基于荧光的微生物快速检测
基于荧光的检测方法可在整个生产过程中加快微生物质量控制。提前获得微生物检测结果的能力使人们能够跟上需求增长的步伐,并在产品受到污染时采取纠正措施。Milliflex® Quantum 系统是一种易于使用、非破坏性、基于荧光染色的系统,可确保获得一致、准确的微生物结果,同时缩短获得结果的总体时间。
用于快速微生物检测的荧光染色
Milliflex® Quantum 系统基于膜过滤和荧光染色,可快速检测微生物。过滤和培养后,将试剂涂抹到膜上,用荧光标记对任何可存活和可培养的微生物进行染色。该反应需要活跃的微生物新陈代谢来酶解非荧光底物。一旦在细胞内裂解,底物就会释放出游离的荧光色素进入微生物细胞质(图 1)。随着荧光素在细胞内的积累,信号自然会被放大。然后,细胞暴露在荧光染料的激发波长下,这样就可以目测计数了。
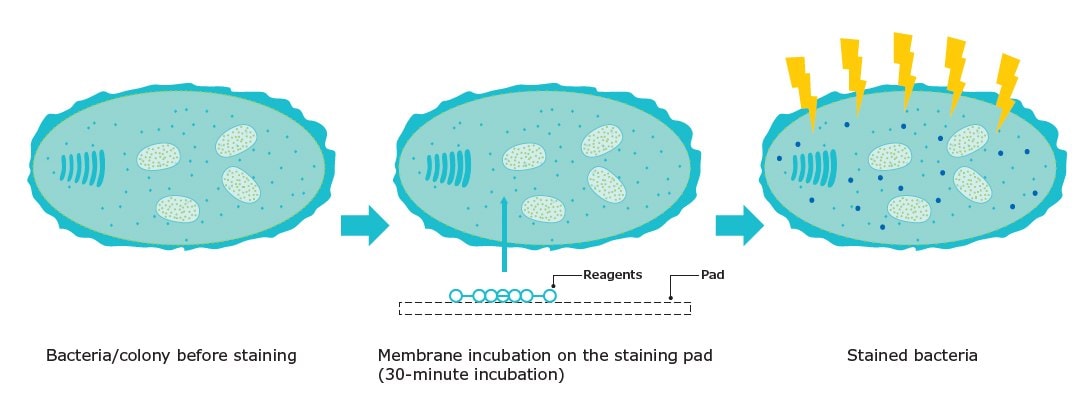
图 1.荧光染色原理。

- 大多数微生物的检测时间为 8 - 48 小时
- 与任何 ID 技术兼容
- 工作流程简单,易于使用,只需少量培训
- 结果可与药典方法相媲美,便于 Milliflex® Quantum 验证
- 硬件紧凑,占地面积小
- 系统经济耐用
1. 样品制备
Milliflex® Quantum 方法使用一种装置进行样品制备,可确保得到一致、可靠的结果。用即用型 Milliflex Oasis® 过滤装置过滤所需体积的样品。
2.荧光染色
将滤膜转移到浸有荧光试剂的垫子上,培养 30 分钟。
3.计算CFU
通过Milliflex®量子阅读器的窗口计算荧光菌落,或使用摄像头在电脑屏幕上查看菌落。
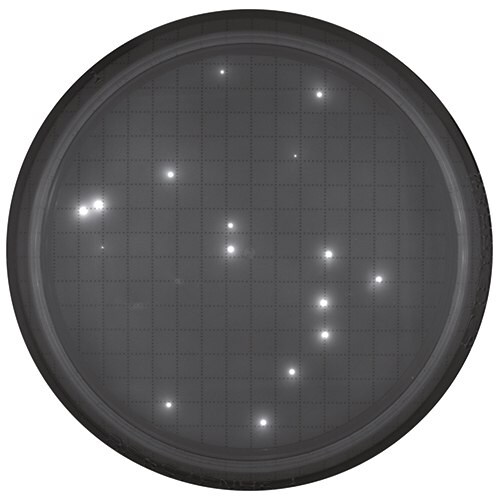
染色后观察平板。阅读器中的膜视图。
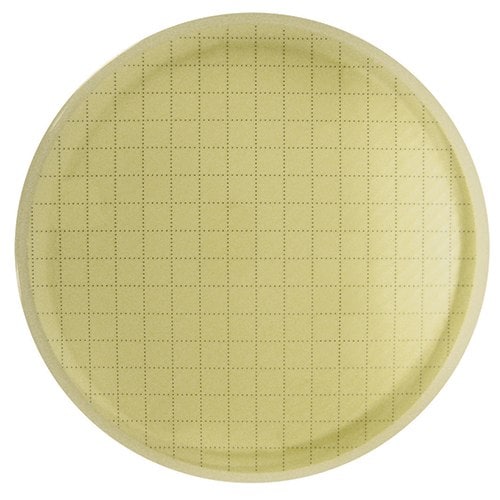
阅读器外看不到 CFU。
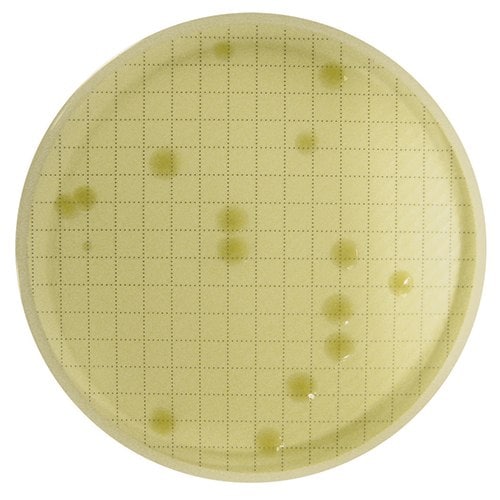
重新培养后,肉眼即可看到 CFU。
作为参考,此处显示的是使用 Milliflex® Quantum 系统检测的过程中非无菌水样品。检测结束后,将膜重新培养以进行充分生长和鉴定。
4.重新培养
重新培养以鉴定微生物。将滤膜放在预装琼脂培养基盒中再培养。收集并分离微生物,使用任何现有的 ID 方法进行鉴定。
Milliflex® Quantum Spot Counter 软件
Milliflex Quantum 相机与 Milliflex® Quantum Spot Counter 软件相结合,使样品读取更加方便,并可跟踪记录,优化微生物质控实验室。 在此下载软件。

瑞士 MGP 咨询公司总经理 Marcel Goverde
"Milliflex® Quantum 作为传统生物负载测试的快速替代方法获得成功验证"

Dr. Tim Sandle
博士、
Read the study by Tim Sandle
观看点播网络研讨会
相关产品
Milliflex® 量子系统
Milliflex Oasis® 过滤泵
Milliflex® Quantum 硬件和附件
Milliflex® Quantum 耗材包
Milliflex® 量子服务
如要继续阅读,请登录或创建帐户。
暂无帐户?