Thin-Layer Chromatographic Identification of Curcuminoids as per USP Monograph Using the TLC Explorer
Abstract
This study utilizes the TLC Explorer to identify curcuminoids by TLC, following USP monograph guidelines. The system enhances TLC efficiency through automation and multiple illumination modes, accurately distinguishing curcumin, demethoxycurcumin, and bisdemethoxycurcumin. Results confirm the suitability of the TLC Explorer for curcuminoid analysis.
Section Overview
Introduction
Curcuminoids are bioactive compounds present in the roots of turmeric (Curcuma longa), a spice widely used in Asian cuisines. These compounds include curcumin, demethoxycurcumin, and bisdemethoxycurcumin (Figure 1).
Known for their potential health benefits, curcuminoids possess anti-inflammatory, antioxidant, and anticancer properties.1 They are often researched for their impact on various health issues, such as arthritis, cardiovascular diseases, and specific cancers. Typically, curcuminoids are consumed through turmeric supplements, extracts, or as part of the diet from turmeric spice.2
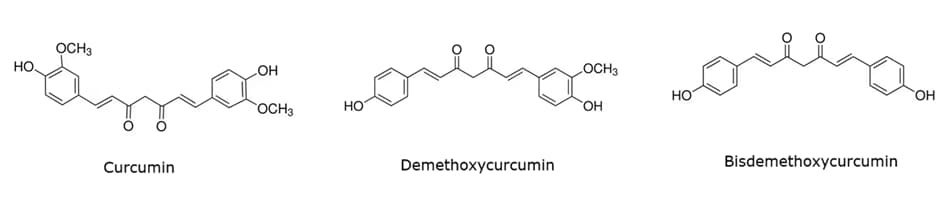
Figure 1.Chemical structures of curcumin, demethoxycurcumin, and bisdemethoxycurcumin.
The United States Pharmacopeia (USP) monograph for curcuminoids identifies thin layer chromatography (TLC) as one method for testing identification.3 TLC is frequently referenced in pharmacopeial methods for identity testing. High-Performance Thin Layer Chromatography (HPTLC), a high-performance version of TLC and often used with automation, is a robust, reliable, rapid, and cost-effective technique used for the qualitative and quantitative analysis of pharmaceutical compounds. This method produces chromatographic fingerprints that can be visualized and stored as electronic images.4,5
This application note presents the identification test for curcuminoids, including curcumin, demethoxycurcumin, and bisdemethoxycurcumin, as specified in the USP monograph, performed using the new TLC Explorer documentation system (Figure 2).

Figure 2.TLC Explorer.
The TLC Explorer documentation system enables the digital and automated evaluation of TLC plates, enhancing the efficiency and accuracy of thin layer chromatography analysis. The device offers three illumination modes using LED light sources—white light (VIS), UV-A (366 nm), and UV-C (254 nm) – for the detection and fast measurement of the compounds of interest. The software offers special features like automated track recognition, simultaneous measurement of multiple plates and background signal correction. Overall, the TLC Explorer offers accurate TLC imaging for reliable densitometric measurements, enabling quantitative analysis and reliable data interpretation.
Experimental
Reagent Preparation
- Mobile phase: Mix toluene and glacial acetic acid in a ratio of 4:1, v:v.
- Derivatization reagent: Add 10 mL of glacial acetic acid and 5 mL of sulfuric acid to 85 mL of ice-cold methanol slowly. Mix and allow the mixture to cool to room temperature. Then add 0.5 mL of p-anisaldehyde and mix well.
Standard Preparation
- Curcuminoids standard solution (1 mg/mL): Weigh and dissolve 5 mg of USP Curcuminoids RS in 5 mL of methanol.
- Curcumin standard solution (1 mg/mL): Weigh and dissolve 5 mg of curcumin in 1 mL of methanol.
- Demethoxycurcumin standard solution (1 mg/mL): Weigh and dissolve 5 mg of demethoxycurcumin in 5 mL of methanol.
- Bisdemethoxycurcumin standard solution (1 mg/mL): Weigh and dissolve 5 mg of bisdemethoxycurcumin in 5 mL of methanol.
Sample Preparation
Test solutions I + II: Weigh 5 mg of curcuminoids in 5 mL of methanol. Sonicate for 10 minutes and centrifuge at 3000 rpm for 5 minutes. The resulting supernatant solution contains 1 mg/mL of curcuminoids.
Instrument Parameters
Results
The identification of curcuminoid extract performed according to USP monograph prior to derivatization under visible/white light (VIS) and 366 nm (here in addition also under 254 nm for comparison) on the TLC Explorer is demonstrated in Figure 3, and after derivatization under VIS and 366 nm is demonstrated in Figure 4. The derivatized chromatogram of the standard solutions under UV 366 nm shows three bands in the order of increasing Rf: an orange band due to bisdesmethoxycurcumin, an orange band due to desmethoxycurcumin, and a red band due to curcumin, as described in the monograph. Under white light, the two lower bands appear orange, while the topmost band is reddish-pink. Table 2 summarizes the obtained chromatographic results.
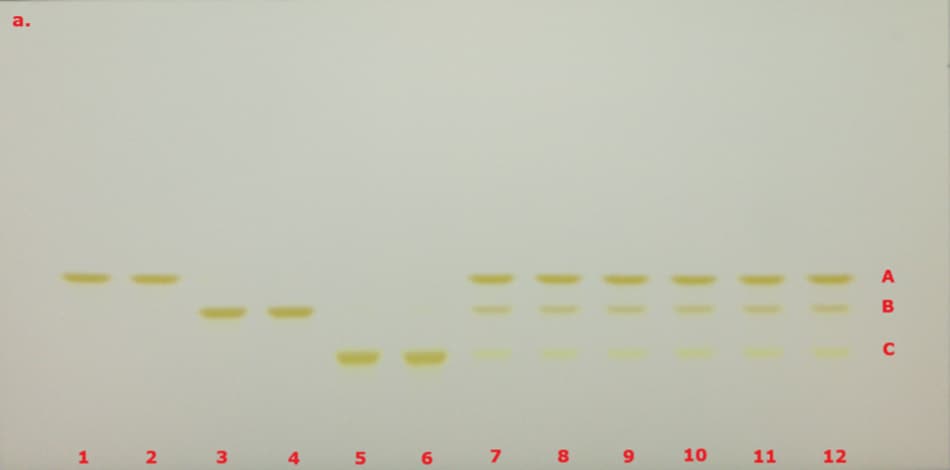
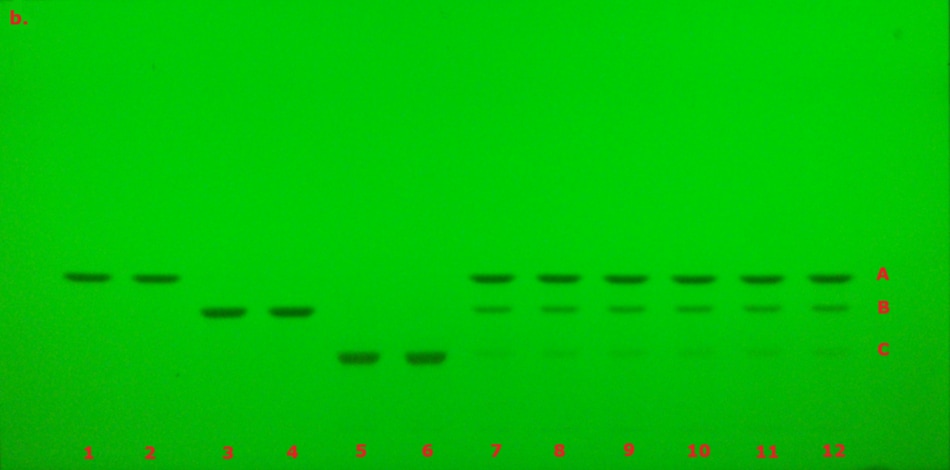
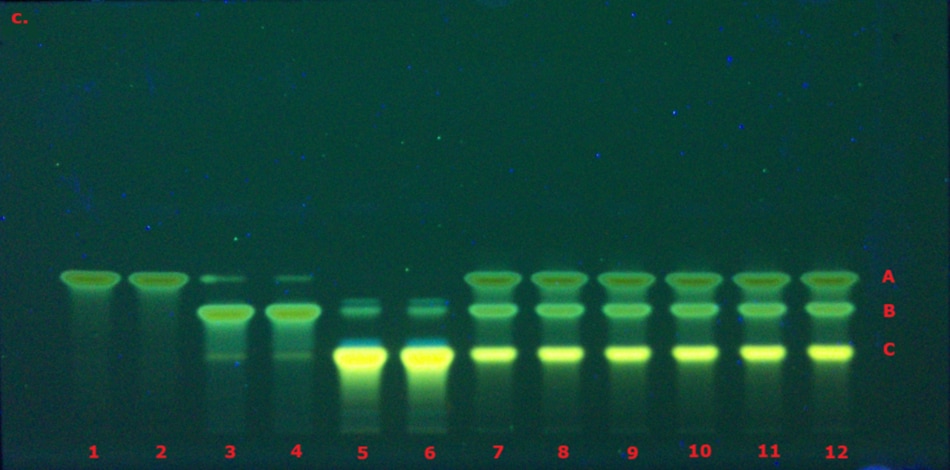
Figure 3. TLC chromatogram demonstrating the identification of curcuminoid extract prior to derivatization under VIS (a), UV 254 nm (b) and UV 366 nm (c) by the TLC Explorer. Band IDs: curcumin (A), demethoxycurcumin (B), and bisdemethoxycurcumin (C). Track allocation see Table 2.
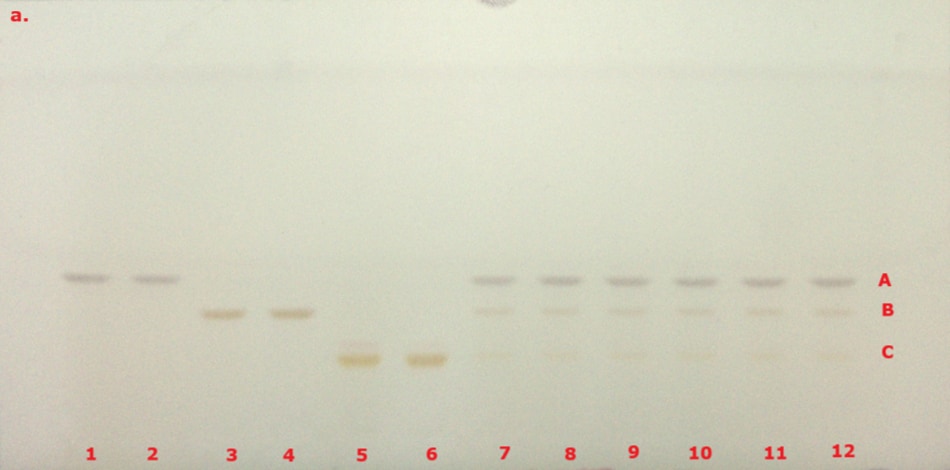
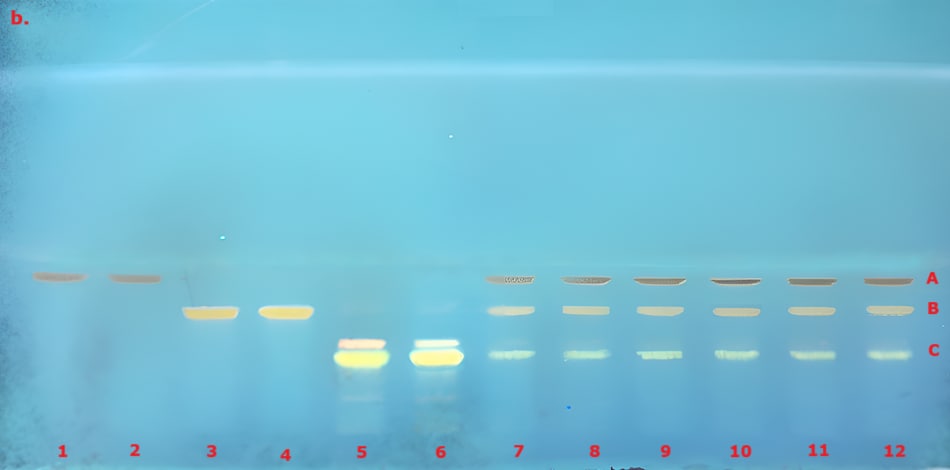
Figure 4. TLC chromatogram demonstrating the identification of curcuminoid extract after derivatization under VIS (a) and UV 366 nm (b) by the TLC Explorer. Band IDs: curcumin (A), demethoxycurcumin (B), and bisdemethoxycurcumin (C). Track allocation see Table 2.
Conclusion
The derivatized chromatogram of the test solutions reveals two orange bands and one red band, which closely match the position and color of those found in the curcuminoid standard solution under VIS and UV 366 nm as described in the USP monograph. Under white light, the two lower orange bands and the upper darker red band correspond to bisdemethoxycurcumin, demethoxycurcumin, and curcumin in the curcuminoid standard solution, arranged in order of increasing retention factor.
This application note demonstrates that the TLC Explorer documentation system serves as an efficient TLC visualizer, enabling data capture, track identification, and Rf value calculation.
Find more information on the TLC Explorer Documentation System.
References
如要继续阅读,请登录或创建帐户。
暂无帐户?