Dextran and Polysaccharides Overview
Dextran
Historically, dextrans had been long recognized as contaminants in sugar processing and other food production. The formation of dextran in wine was shown by Pasteur to be due to the activity of microbes.1 The name dextran was created by Scheibler in 1874, who demonstrated dextran was a carbohydrate with the formula (C6H10O6)n and a positive optical rotation.2
Dextrans are polysaccharides with molecular weights ≥1,000 Dalton, which have a linear backbone of α-linked d-glucopyranosyl repeating units. Three classes of dextrans can be differentiated by their structural features. The pyranose ring structure contains five carbon atoms and one oxygen atom. Class 1 dextrans contain the α(1→6)-linked d-glucopyranosyl backbone modified with small side chains of d-glucose branches with α(1→2), α(1→3), and α(1→4)-linkage (see Figure 1). The class 1 dextrans vary in their molecular weight, spatial arrangement, type and degree of branching, and length of branch chains,3-5 depending on the microbial producing strains and cultivation conditions.6,7 Isomaltose and isomaltotriose are oligosaccharides with the class 1 dextran backbone structure. Class 2 dextrans (alternans) contain a backbone structure of alternating α(1→3) and α(1→6)-linked d-glucopyranosyl units with α(1→3)-linked branches. Class 3 dextrans (mutans) have a backbone structure of consecutive α(1→3)-linked d-glucopyranosyl units with α(1→6)-linked branches. One and two-dimensional NMR spectroscopy techniques have been utilized for the structural analysis of dextrans.8
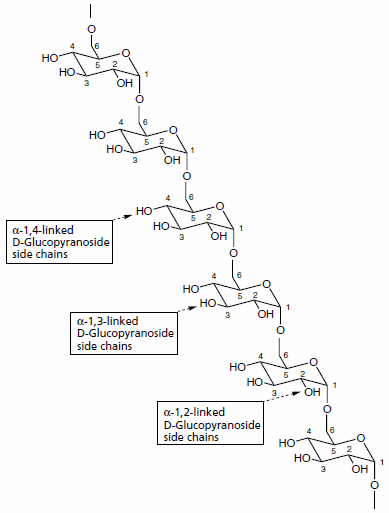
Figure 1.General structure of class 1 dextrans consisting of a linear backbone of α(1→6)-linked d-glucopyranosyl repeating units. The dextran may have branches of smaller chains of d-glucose linked to the backbone by α(1→2)- , α(1→3)- or α(1→4)- glycosidic bonds.
The secretion of dextrans provides an opportunity for bacteria to modulate adhesion, e.g. in tooth decay, by having a softer or more rigid bacterial cell surface, depending on the polysaccharide itself and the pH and ionic strength. Low bacterial adhesion occurs at low salt conditions with more rigid polysaccharides and a softer surface, while high bacterial adhesion is obtained with more flexible polysaccharides and a rigid bacterial surface. Polymer elasticity is important for structural integrity. and the pyranose ring is the structural unit controlling the elasticity of the polysaccharide. This elasticity results from a force-induced elongation of the ring structure and a final transition from a chair-like to a boat-like conformation of the glucopyranose ring, which plays an important role in accommodating mechanical stress and modulating ligand binding in biological systems.9 Laboratory experiments have demonstrated that cleavage of the pyranose rings of dextran, amylose, and pullulan convert these different polysaccharide chains into similar structures where all the bonds of the polymer backbone can rotate and align under force. After ring cleavage, single molecules of dextran, amylose, and pullulan display identical elastic behavior as measured by atomic force microscopy.
Dextrans are found as bacterial extracellular polysaccharides. They are synthesized from sucrose by beneficial lactic acid bacteria, such as Leuconostoc mesenteroides and Lactobacillus brevis, but also by the dental plaque-forming species Streptococcus mutans. Bacteria employ dextran in biofilm formation10 or as protective coatings, e.g., to evade host phagocytes in the case of pathogenic bacteria.11
The physical and chemical properties of purified dextrans vary depending on the microbial strains from which they are produced and by the production method, but all are white and tasteless solids. Dextrans have high water solubility and the solutions behave as Newtonian fluids. Solution viscosity depends on concentration, temperature, and molecular weight, which have a characteristic distribution.
The long history of the safety of dextrans has allowed them to be used as additives to food and chemicals, and in pharmaceutical and cosmetics manufacturing.12 Dextrans have been investigated for the targeted and sustained delivery of drugs, proteins, enzymes, and imaging agents.13 In medicine, clinical grades of dextrans with a molecular weight range of 75-100 kDa have been used as blood-plasma volume expanders in transfusions.14 Other applications include the use of dextrans with polyethylene glycol as components of aqueous two-phase systems for the extraction of biochemicals. The hydroxyl groups present in dextran offer many sites for derivatization, and these functionalized glycoconjugates represent a largely unexplored class of biocompatible and environmentally safe compounds.
Cross-linked dextran beads are widely used for chromatography in biochemical research and industry. The classic application of cross-linked dextrans is as gel filtration media in packed-bed columns for the separation and purification of biomolecules with molecular weights in the range of 0.7-200 kDa.15-17 Ion exchange chromatography utilizes dextran that has been derivatized with positively or negatively charged moieties such as carboxymethyl (CM), diethylaminoethyl (DEAE), diethyl(2-hydroxypropyl) aminoethyl (QAE), and sulfopropyl (SP).
We offer a large variety of dextrans with high polydispersity and dextran molecular weight standards with low polydispersity (Mw/Mn values close to 1.0).
Other Polysaccharides
Pullulans are structural polysaccharides primarily produced from starch by the fungus Aureobasidium pullulans.18,19 Pullulans are composed of repeating α(1→6)-linked maltotriose (D-glucopyranosyl-α(1→4)-D-glucopyranosyl-α(1→4)-D-glucose) units with the inclusion of occasional maltotetraose units.20 Diffusion-ordered NMR spectroscopy has been used to achieve a simple estimation of the molecular weight of pullulan.21 The solution properties of pullulan in water have been studied, and it was confirmed that pullulan molecules behave as random coils in aqueous solution.22
Dextrins are composed of D-glucopyranosyl units but have shorter chain lengths than dextrans. They start with a single α(1→6) bond, but continue linearly with α(1→4)-linked D-glucopyranosyl units. Dextrins are usually mixtures derived from the hydrolysis of starch and have found widespread use in the food, paper, textile, and pharmaceutical industries.
Dextran sulfates are derived from dextran via sulfation. They have become indispensable components in many molecular biology techniques, including the transfer of large DNA fragments from agarose gels and rapid hybridization,23 precipitation procedures for the quantitation of high-density lipoprotein cholesterol,24 and inhibition of virion binding to CD4+ cells.25
Materials
References
如要继续阅读,请登录或创建帐户。
暂无帐户?