Analysis of Catechins in Green Tea (Decaffeinated) by TLC Using TLC Explorer
Abstract
Catechins in decaffeinated green tea were separated and quantified on a silica gel 60 F254 TLC plate aligned to the USP monograph method. Analysis and documentation were performed using the TLC Explorer instrument. The method performance was assessed against the system suitability criteria specified in the monograph for identification and was subsequently extended to the quantitative determination of a representative catechin in powdered decaffeinated green tea extract.
Section Overview
Introduction
Camellia sinensis is an evergreen shrub or small tree belonging to the family Theaceae, the leaves, leaf buds, and stems of which are used for tea production. Commonly referred to as the tea plant, tea shrub, or tea tree,1 tea derived from Camellia sinensis is categorized into four major types according to the processing method applied, namely green, black, white, and oolong tea.
Green tea is produced from the leaves of Camellia sinensis without fermentation, thereby limiting the oxidation of its polyphenolic constituents. The predominant polyphenols present in green tea are catechins.2 These naturally occurring polyphenolic phytochemicals are recognized for their potential health benefits, including favorable effects on metabolic health, such as obesity related to high-fat diets and type II diabetes, as well as a reduced risk of coronary diseases.3 Additional beneficial effects of green tea have been reported for various types of cancer as well as cardiovascular and liver diseases, effects that are largely attributed to the catechin content of green tea.4
Decaffeinated green tea is obtained from tea leaves subjected to a decaffeination process to eliminate caffeine. When a natural water-based decaffeination process is applied, the tea retains approximately 95% of its antioxidants, thereby preserving most of the health benefits of regular green tea. Consequently, naturally processed decaffeinated tea retains most of the health-related benefits while minimizing caffeine content.5 As the decaffeination process may impact the polyphenol composition, determination of catechin content in the final product is of interest.
The catechins specified in the USP monograph for powdered decaffeinated green tea extract include (−)-epigallocatechin-3-O-gallate, (−)-epigallocatechin, (−)-epicatechin-3-O-gallate, and (−)-epicatechin (Figure 1).6
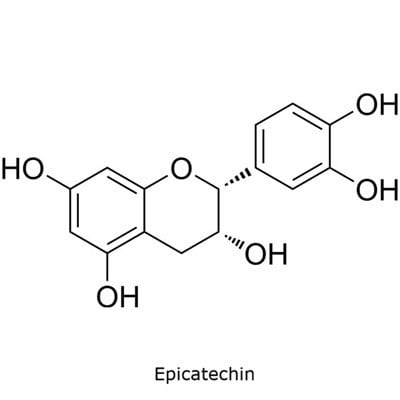
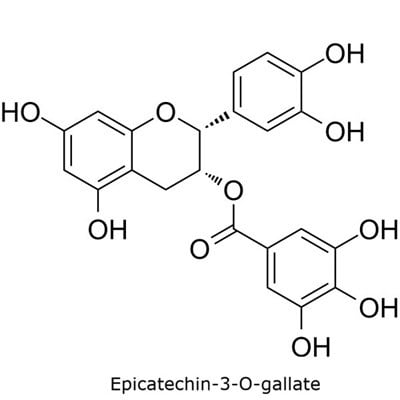
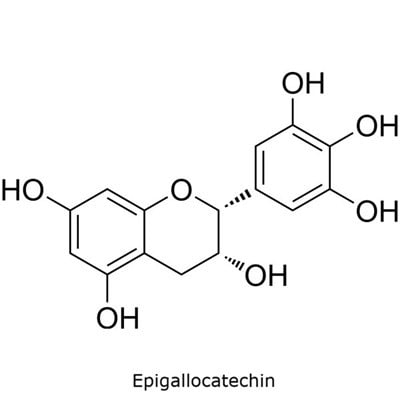
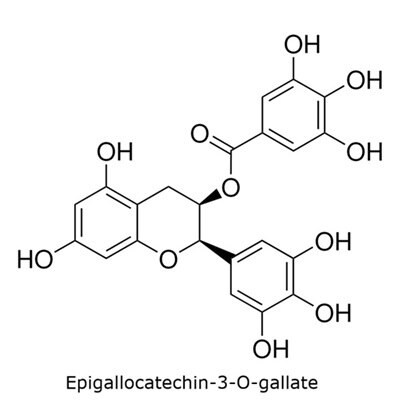
Figure 1. TLC Explorer used for the analysis of catechins in decaffeinated green tea.
In this study, the separation and quantification of the most intense chromatographic band corresponding to (-)-epigallocatechin-3-O-gallate in a powdered decaffeinated green tea extract are performed using a silica gel 60 F254, 20 x 20 cm, thin layer chromatography (TLC) plate. While the USP monograph for decaffeinated green tea specifies HPTLC as the method for qualitative analysis (identification) and HPLC as the quantitation method, the present work extends the TLC-based approach beyond identification to enable quantification of (-)-epigallocatechin-3-O-gallate, in the presence of the sample matrix and other catechins naturally occurring in the decaffeinated green tea.
The USP monograph for decaffeinated green tea recommends the use of an HPTLC plate with an average particle size of 5 µm for qualitative determination of catechins. In contrast, this study uses a TLC plate with a larger particle size of 10-12 µm and demonstrates its applicability for both catechin identification and quantitative determination of epigallocatechin-3-O-gallate as a representative analyte.
The identification and imaging of catechin compounds together with the quantification of (-)-epigallocatechin-3-O-gallate were performed by video densitometry with the TLC Explorer instrument. This analysis and documentation system (Figure 2) provided an easy-to-use and reliable approach for TLC plate imaging and data handling in analytical routines, method development, in-process control, or daily research activity.

Figure 2.TLC Explorer used for the analysis of catechins in decaffeinated green tea.
The TLC Explorer documentation system enables digital and automated evaluation of TLC plates, enhancing the efficiency and accuracy of thin-layer chromatography analyses. The instrument offers three illumination modes based on LED light sources, namely white light (VIS), UV-A (366 nm), and UV-C (254 nm), allowing detection and rapid measurement of compounds of interest. The associated software offers special features like automated track recognition, simultaneous measurement of multiple plates, and background signal correction. The TLC Explorer offers accurate TLC imaging to support video densitometric measurements, thereby enabling quantitative analysis and reliable data interpretation.
The method developed using the TLC Explorer instrument was partially validated as per the ICH Q2 R2 guidelines.7
Experimental
Reagent, Standard and Sample Preparations
- Immersion reagent: Dissolve 140 mg of Fast Blue B salt in 10 mL of water, then add 140 mL of methanol and 50 mL of dichloromethane.
- Diluent: Ethyl alcohol/water (4:1 v:v)
- Standard solution USP RS (4 mg/mL): Weigh and transfer 40 mg of USP powdered decaffeinated green tea extract RS into a 10 mL volumetric flask. Add 5 mL of diluent and sonicate for 10 min with intermittent shaking. Make up to volume with diluent, mix thoroughly, and centrifuge. Use the clear supernatant.
- (-)-Epigallocatechin-3-O-gallate reference solution (2 mg/mL): Weigh and transfer 10 mg of (-)-epigallocatechin-3-O-gallate RS into a 5 mL volumetric flask. Add 4-5 mL of diluent and sonicate for 10 min with intermittent shaking. Mix thoroughly and make up to volume with diluent.
- (-)-Epigallocatechin reference solution (2 mg/mL): Weigh and transfer 10 mg of (-)-epigallocatechin RS into a 5 mL volumetric flask. Add 4-5 mL of diluent and sonicate for 10 min with intermittent shaking. Mix thoroughly and make up to volume with diluent.
- (-)-Epicatechin-3-O-gallate reference solution (2 mg/mL): Weigh and transfer 10 mg of (-)-epicatechin-3-O-gallate RS into a 5 mL volumetric flask. Add 4-5 mL of diluent and sonicate for 10 min with intermittent shaking. Mix thoroughly and make up to volume with diluent.
- (-)-Epicatechin reference solution (2 mg/mL): Weigh and transfer 10 mg of (-)-epicatechin) RS into a 5 mL volumetric flask. Add 4-5 mL of diluent and sonicate for 10 min with intermittent shaking. Mix thoroughly and make up to volume with diluent.
Sample solution commercial extract: Weigh and transfer 40 mg of powdered decaffeinated green tea extract into a 10 mL volumetric flask. Add 5 mL of diluent and sonicate for 10 min with intermittent shaking. Make up to volume with diluent, mix thoroughly, and centrifuge. Use the clear supernatant.
TLC Method
In deviation from the USP monograph, which specifies the use of an HPTLC plate with a particle size of 5 µm, the separation was performed on a silica gel 60 F254 TLC plate on aluminum support with a particle size of 10-12 µm (Table 1). After development and drying, the plate was dipped in the immersion reagent solution and subsequently examined under white light.
Table 2. Applied spots and sample application volume for identification (Plate A)
Table 3. Applied spots and application volume for quantification, linearity, and recovery (Plate B)
Results and Discussion
Identification of Catechins
Identification of catechins was achieved by individual application of decaffeinated green tea extract (USP RS), decaffeinated green tea extract sample, and four reference standards, namely (-)-epigallocatechin-3-O-gallate, (-)-epigallocatechin, (+)–epicatechin-3-O-gallate, and (-)-epicatechin, onto a silica gel 60 F254 TLC plate. The resulting chromatograms obtained under UV (254 nm) and white light are shown in Figure 3. Details of applied spots and application volumes are provided in Figure 3 and Table 1. The retardation factor (Rf) values for the compounds in both standard and sample solutions are listed in Table 4.
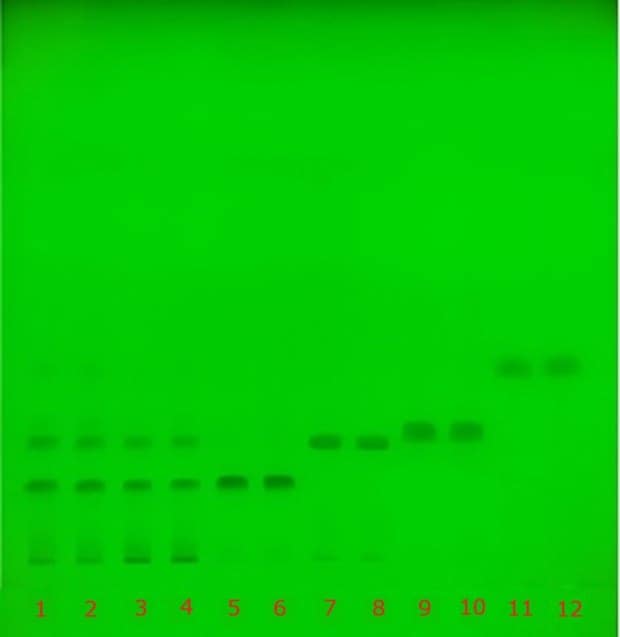
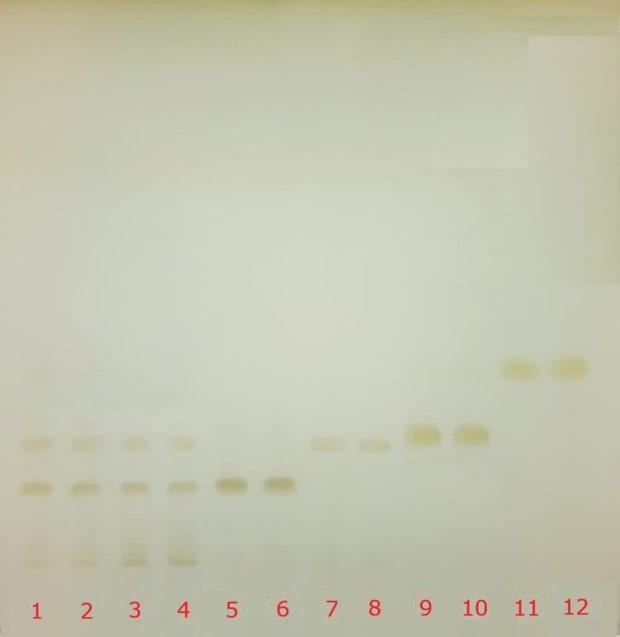
Figure 3. TLC chromatograms (Plate A) recorded under UV (254 nm, green) and visible light (white) for the identification of catechins in powdered decaffeinated green tea extract. Tracks 1 & 2 correspond to decaffeinated green tea standard solution (USP RS), tracks 3 & 4 to decaffeinated green tea extract sample solution, tracks 5 & 6 to (-)-epigallocatechin-3-O-gallate, tracks 7 & 8 to (‑)-epigallocatechin, tracks 9 & 10 to (+)–epicatechin-3-O-gallate, and tracks 11 & 12 to (-)-epicatechin.
Comparison to USP Monograph Criteria
The USP monograph specifies the identification of key catechins by the presence of four prominent brownish-orange bands observed under white light with approximate Rf values on an HPTLC plate with a particle size of 5 µm. In the present study, the Rf values obtained using a TLC plate with a particle size of 10-12 µm were compared with the monograph criteria and are summarized in Table 5. A comparable band pattern was observed. The monograph acceptance criterion, requiring correspondence between the key bands of the sample solution and those of the standard solution, was fulfilled.
Calibration and Recovery
Quantification, linearity, and recovery studies were conducted using reference standards and over-spiked sample spots applied on Plate B, as described in Table 3. The resulting chromatograms were visualized under white light (Figure 4) and used for calibration, quantification, and recovery assessment. Additional documentation was obtained under UV light at 254 nm and 366 nm (Figure 5).
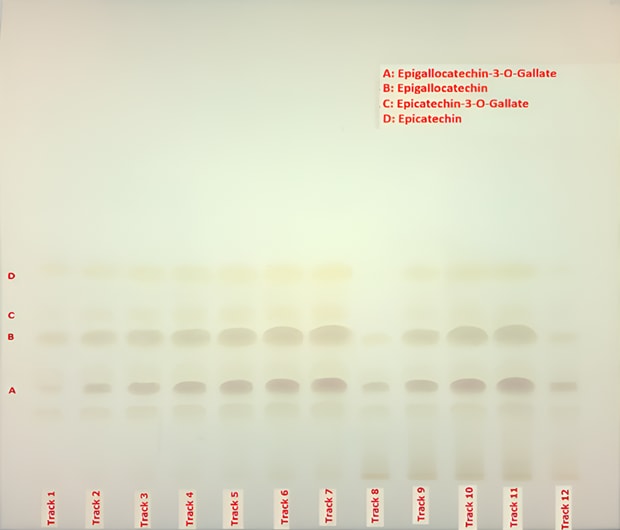
Figure 4. TLC chromatogram (Plate B) for catechins present in powdered decaffeinated green tea extract recorded under visible light. A. (-)-epigallocatechin-3-O-gallate, B. (‑)-epigallocatechin, C. (+)–epicatechin-3-O-gallate, D. (-)-epicatechin.
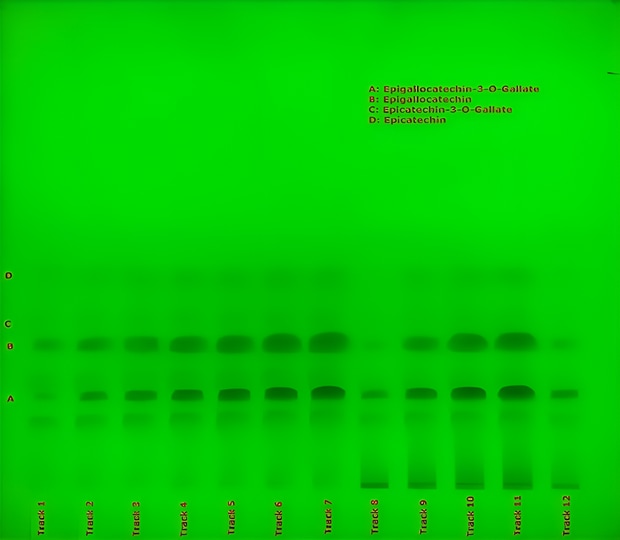
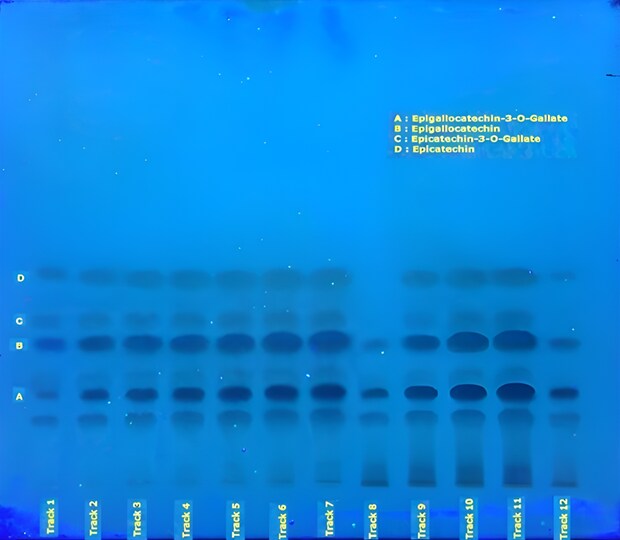
Figure 5. TLC chromatogram obtained on Plate B for catechins present in powdered decaffeinated green tea extract recorded under UV 254 nm (green) and 366 nm (blue). A. (‑)-epigallocatechin-3-O-gallate, B. (‑)-epigallocatechin, C. (+)–epicatechin-3-O-gallate, D. (-)-epicatechin.
Calibration
Calibration was performed using the most intense chromatographic band corresponding to (-)-epigallocatechin-3-O-gallate (B) observed on the TLC plate under white light. A calibration range from 1 µg per spot to 7 µg per spot was established by applying increasing volumes of the standard stock solution, as described in Table 3. A representative densitogram for (-)-epigallocatechin-3-O-gallate (3 µg/spot) is shown in Figure 6. This approach was used to determine the peak areas of (-)-epigallocatechin-3-O-gallate in all samples listed in Table 6. The resulting calibration curve, shown in Figure 7, demonstrated a polynomial fit with a R2 value of 0.9968.
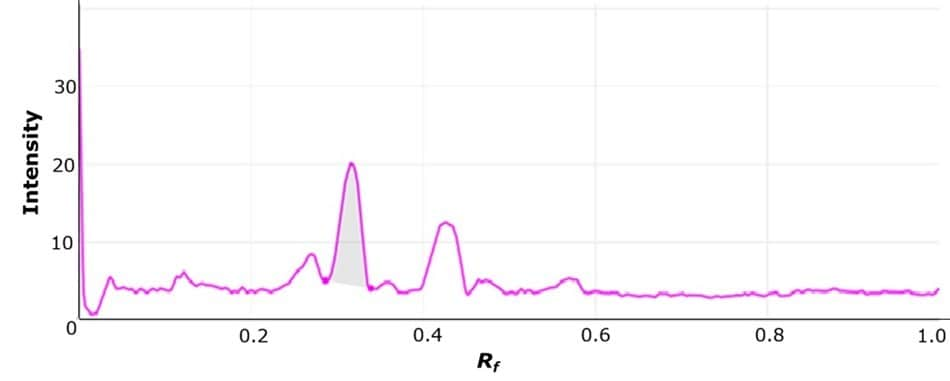
Figure 6.Representative densitogram recorded under white light for the standard solution applied at 3 µg of (-)-epigallocatechin-3-O-gallate per spot (for details see Table 3).
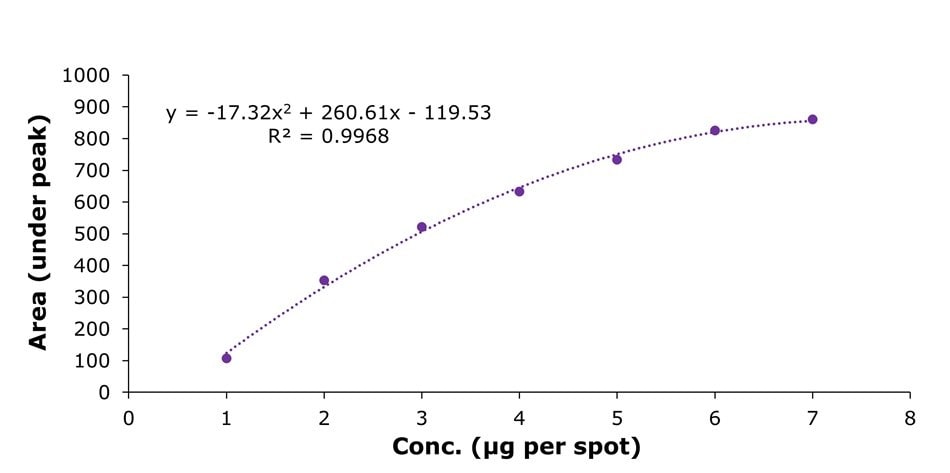
Figure 7.Calibration curve using a polynomial function in the concentration range of 1 to 7 µg per spot of (-)-epigallocatechin-3-O-gallate.
Recovery
Recovery was evaluated using commercial decaffeinated tea extract sample spots that were over-spiked with 2, 5, and 7 µg/spot of analyte. The results of the recovery study are shown in Table 7 (tracks 9-11). Analysis of the unspiked sample indicated that the sample spiked at 7 µg per spot (Plate B, Track 11) exceeded the applicable calibration range of 1-7 µg/spot. Consequently, this data point was excluded from recovery calculations. Nevertheless, the higher concentration spot showed consistency with respect to the Rf value. The recoveries obtained for the samples over-spiked with 2 µg and 5 µg per spot were 92.0% and 95.7%, respectively.
Commercial Extract Sample Result
For the unspiked commercial sample extract spot (Table 7, Tack 8) a content of 1.9 µg per spot of (-)-epigallocatechin-3-O-gallate was determined with an applied sample volume of 1 µL. This corresponds to a concentration of 475 mg/g in the original extract powder.
Conclusion
Using TLC plates along with the TLC Explorer instrument and with reference to the USP monograph method for powdered decaffeinated green tea extract, the acceptance criteria for catechin identification were fulfilled using TLC plated with a particle size of 10-12 µm. The chromatogram of the sample solution exhibited major bands comparable in color and size to those observed for the standard solution.
Although the USP monograph describes the use of HPTLC solely for qualitative identification of catechins, the method developed in this study was extended to enable quantitative determination of the most intense band corresponding to (-)-epicatechin-3-O-gallate. Calibration demonstrated a good correlation over the range of 1 to 7 µg per spot with R2 values greater than 0.996. Recovery studies performed by over-spiking sample spots yielded recoveries between 92% to 95.7%.
Overall, the presented method demonstrates that the combination of TLC plates and the TLC Explorer system is suitable for both qualitative identification and quantitative determination of catechins in powdered decaffeinated green tea extract.
Solvents, Reagents, and Consumables
Reference Materials
References
如要继续阅读,请登录或创建帐户。
暂无帐户?