Identification of Glycyrrhizic Acid in Licorice acc. to USP and Quantification Using the TLC Explorer
Abstract
A thin-layer chromatographic method for the identification of glycyrrhizic acid in licorice was established in accordance with the United States Pharmacopeia (USP) monograph. The TLC Explorer system was utilized for improved digital documentation, automated assessment, and quantitative analysis, thereby broadening the use of TLC for this application. Effective chromatographic separation and documentation were achieved, and glycyrrhizic acid was successfully detected in licorice samples. The results demonstrated that the TLC Explorer system provides a reliable platform for TLC-based analysis and documentation.
Section Overview
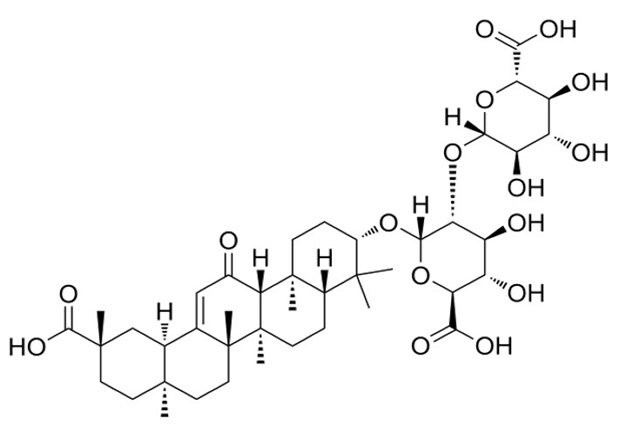
Figure 1.Chemical structure of glycyrrhizic acid (glycyrrhizin).
Plants produce various secondary metabolites that can serve a wide variety of medicinal functions beneficial to humans. Licorice (Glycyrrhiza glabra, G. uralensis) has been extensively used in Eastern and Western medicine for the treatment of conditions, including the common cold, fever, liver ailments, gastric ulcers, asthma, bronchitis, Addison’s disease, and rheumatoid arthritis. Additionally, its use as a laxative, antitussive, and expectorant has also been documented.1
Glycyrrhizic acid (Figure 1), also known as glycyrrhizin, is a triterpenoid saponin found in the roots, rhizomes, and stolon of licorice plant. In the gastrointestinal tract, glycyrrhizin is metabolized to glycyrrhetinic acid, which has been identified as the primary contributor to the pharmacological and biological activities associated with licorice.2
Thin-layer chromatography (TLC) is designated in the United States Pharmacopeia (USP) monograph for licorice as an identification procedure.3 TLC-based approaches are widely cited in pharmacopeial identity tests. High-Performance Thin-Layer Chromatography (HPTLC), an advanced form of TLC commonly paired with automation, has been established as a robust, reliable, rapid, and cost-effective technique for both qualitative and quantitative analysis of medicinal compounds. Chromatographic fingerprints generated by this technique can be visualized and archived as electronic images.4,5
In the present study, the TLC identification test for glycyrrhizic acid prescribed in the USP monograph was executed using the TLC Explorer documentation system (Figure 2). The system enabled not only standardized digital documentation but also a quantitative assessment of glycyrrhizic acid content to evaluate compliance with the USP requirement for licorice (NLT 2.5% on the dried basis). Three commercially available licorice brands were examined.

Figure 2.TLC Explorer.
The TLC Explorer documentation system enables the digital and automated evaluation of TLC plates, enhancing the efficiency and accuracy of thin layer chromatography analysis. The device offers three illumination modes using LED light sources—white light (Vis), UV-A (366 nm), and UV-C (254 nm) – for the detection and fast measurement of the compounds of interest. The software offers special features like automated track recognition, simultaneous measurement of multiple plates and background signal correction. Overall, the TLC Explorer offers accurate TLC imaging for reliable densitometric measurements, enabling quantitative analysis and reliable data interpretation.
Experimental
Reagent Preparation
Mobile phase: Mix butyl alcohol, acetic acid glacial and water in a ratio of 7:1:2, v:v:v.
Diluent: Mix ethyl alcohol and water in a ratio of 7:3, v:v.
Standard Preparation
- Standard solution 1 for identification (5 mg/mL): Weigh and dissolve 50 mg of glycyrrhizic acid RS in 10 mL of diluent.
- Standard solution 2 for quantification (0.125 mg/mL): Weigh and dissolve 50 mg of glycyrrhizic acid RS in 100 mL of diluent. Dilute 2.5 mL of this solution to 10 mL with diluent.
Sample Preparation
- Samples: Three different commercially available licorice brands.
- Test solutions for identification I + II + III: Weigh and add 2 g of pulverized licorice to 10 mL of diluent. Heat the solution by shaking on a water bath for 5 minutes, cool and filter through a 0.45 µm PVDF syringe filter.
- Test solutions for quantification IV + V + VI: Weigh and add 500 mg of pulverized licorice to 10 mL of diluent. Heat the solution by shaking on a water bath for 5 minutes, cool and filter through a 0.45 µm PVDF syringe filter. Dilute 1 mL of this filtrate to 10 mL with diluent.
Instrument Parameters
Results
Identification
The identification of glycyrrhizic acid in licorice, performed according to the USP monograph and visualized at 254 nm using the TLC Explorer system, is shown in Figure 3. A dark purple zone corresponding to glycyrrhizic acid was observed for both the standard solution and the test solutions under UV 254 nm, in addition to other sample constituents. The chromatographic results obtained from these analyses are summarized in Table 2.
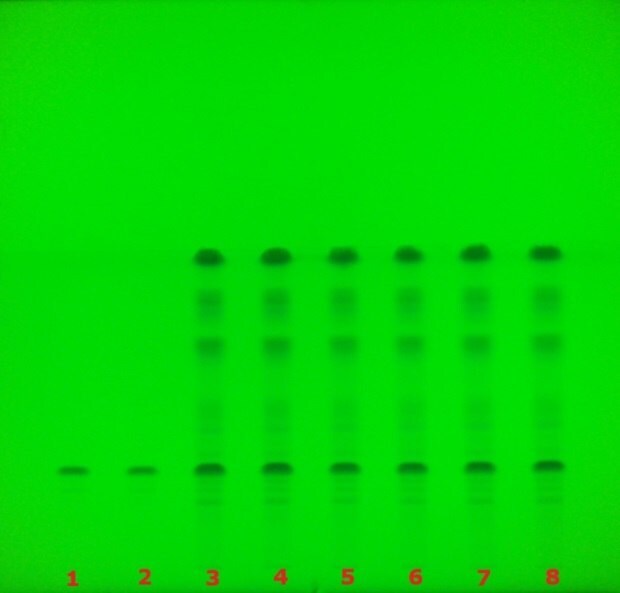
Figure 3.TLC chromatogram showing the identification of glycyrrhizzic acid under UV 254 nm using the TLC Explorer.
Quantification
The quantification of glycyrrhizic acid in three commercial licorice brands was carried out at 254 nm (Figure 4) using the TLC Explorer system with video densitometry. A one-point calibration was applied using the 0.125 mg/mL standard solution, which corresponds to a sample concentration equivalent to 2.5% under the conditions employed. Table 3 summarizes the obtained chromatographic results showing that all three licorice samples contained glycyrrhizic acid at levels exceeding the USP acceptance criterion (NLT 2.5%).

Figure 4.TLC chromatogram for the quantification of glycyrrhizic acid under UV 254 nm as documented by the TLC Explorer system.
Conclusion
The USP monograph–specified identification test for glycyrrhizic acid in licorice was carried out using thin-layer chromatography, and the chromatographic assessment and documentation were performed with the TLC Explorer system. The principal spots observed in the chromatograms of the test solutions exhibited Rf values consistent with the principal spot in the standard chromatogram, fulfilling the requirements of the monograph. In addition to the identity test, the method was extended to a quantitative evaluation of glycyrrhizic acid using the TLC Explorer’s video-densitometric capabilities. The glycyrrhizic acid content of the examined licorice samples was determined to range from 4.32% to 6.94%, exceeding the USP acceptance threshold of not less than 2.5%.
Overall, the study demonstrated that the TLC Explorer system provided reliable chromatographic documentation, track identification, Rf calculation, and video-densitometric quantification for the analysis of glycyrrhizic acid in licorice.
Solvents, Reagents & Reference Materials
References
如要继续阅读,请登录或创建帐户。
暂无帐户?