Hydrosilylation Catalyst
[Cp*Ru(MeCN)3]PF6: A Highly Efficient Hydrosilylation Catalysts
Vinylsilanes are versatile organometallic reagents that participate in a variety of reaction paradigms such as Tamao–Fleming oxidation, olefin metathesis, Pd-catalyzed cross-coupling, protodesilylation, and cycloaddition. Of the available methods for preparation of vinylsilanes, the hydrosilylation of alkynes is the most direct and atom-economical approach (Scheme 1). A number of transition metal catalysts have been devised to execute these reactions in a regio- and stereocontrolled fashion (Figure 1).
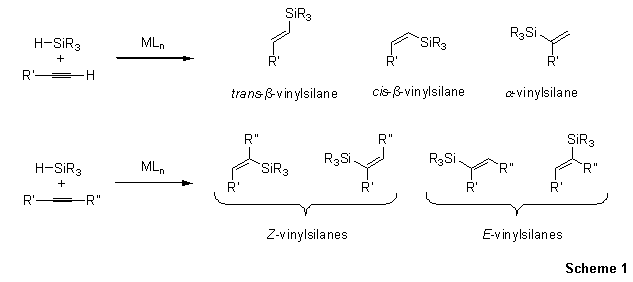
Scheme 1.Hydrosilylation of Alkynes
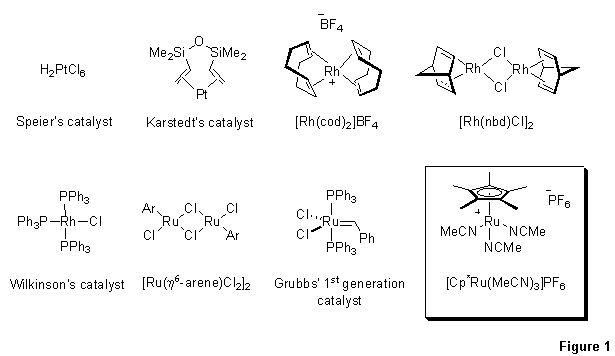
Figure 1.Transition metal catalysts have been devised to execute reactions in a regio- and stereocontrolled fashion.
Methods for hydrosilylation of terminal alkynes were developed some time ago for the preparation of cis- and trans-β-vinylsilanes. Classical Pt-catalysis (Speier’s1 and Karstedt’s2 catalysts), as well as Rh-based catalysis ([Rh(cod)2]BF4 3 and [RhCl(nbd)]2 4), remain powerful methods for synthesis of trans-β-vinylsilanes. Wilkinson’s catalyst was also demonstrated to yield the trans product in polar solvents, with the cis isomer predominating in non-polar media.5 Ru-based catalysts (e.g. [Ru(benzene)Cl2]2 or [Ru(p-cymene)Cl2]2) allow for access to cis-β-vinylsilanes.6 Under certain conditions, Grubbs’ 1st generation catalyst also gives cis products, although the stereo- and regioselectivity of the hydrosilylation is highly dependent on the alkyne, silane, and solvent.7 While there exists a wealth of methods for preparation of linear β-vinylsilanes, until recently there were no general methods for the preparation of 1,1-disubstituted α-vinylsilanes.8 Moreover, although selective intramolecular hydrosilylation of internal alkynes can be achieved,9 a selective intermolecular variant was virtually unknown.10 The Trost group at Stanford University developed a remarkably robust protocol for hydrosilylation of terminal acetylenes to give α-vinylsilanes, relying on the ruthenium(II) catalyst, [Cp*Ru(MeCN)3]PF6.11-14 This catalyst also provides a competent method for regioselective intra- and intermolecular hydrosilylation of internal alkynes, giving exclusively Z-trisubstituted alkenes.
Intermolecular Hydrosilylation: Terminal Alkynes
A diverse set of terminal alkynes underwent rapid and mild hydrosilylation in the presence of [Cp*Ru(MeCN)3]PF6 to give 1,1-disubstituted α-vinylsilanes in good to excellent yield, often with low catalyst loadings (Scheme 2). The reaction is tolerant to a wide range of functional groups including halogens, free alcohols, alkenes, internal alkynes, esters, and amines. Moreover, a breadth of silanes can be used in the reaction with excellent predictability.
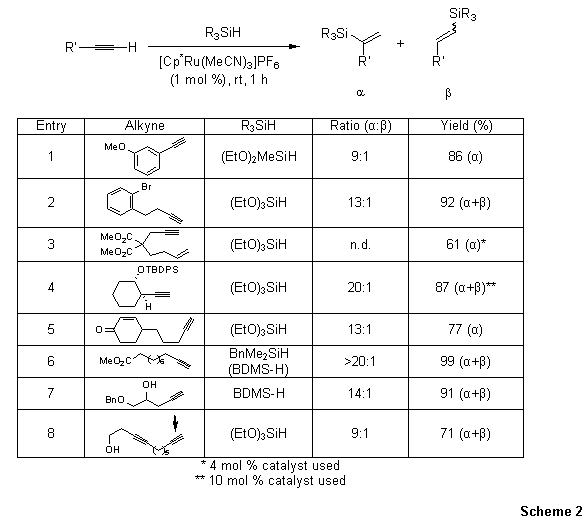
Scheme 2.Intermolecular Hydrosilylation: Terminal Alkynes
Intermolecular Hydrosilylation: Internal Alkynes
As illustrated in Scheme 1, non-selective hydrosilylation of internal alkynes would potentially give four isomeric addition products. The Trost group has demonstrated that hydrosilylation of internal alkynes with [Cp*Ru(MeCN)3]PF6 gives trisubstituted Z-vinylsilanes exclusively, as a result of trans addition of the silane to the alkyne (Scheme 3).11
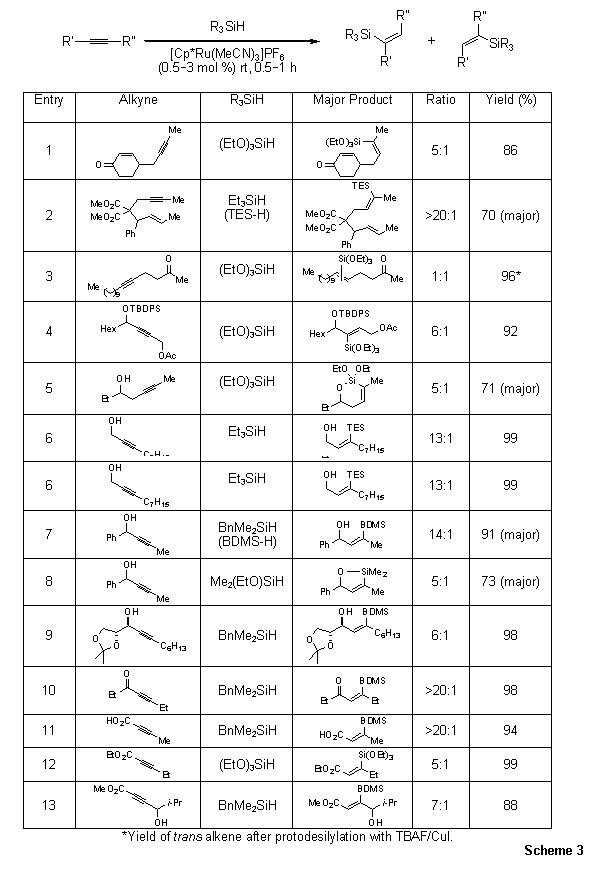
Scheme 3.Intermolecular Hydrosilylation: Internal Alkynes
Importantly, the hydrosilylation reaction displays a high level of regioselectivity. The regioselectivity can be summarized as follows: (i) hydrosilylation of 2-alkynes results in the formation of Z-alkenes with the silyl group occupying the less sterically-demanding position (entries 1 and 2); (ii) for substrates where the alkyne is not in the 2-position, the silyl substituent will occupy the more sterically-demanding position in the Z-alkene (entry 4); (iii) for propargylic, homopropargylic, and bishomopropargylic alcohol substrates, hydrosilylation occurs such that the silyl group resides distal to the hydroxyl functionality of the Z-alkene (entries 5–9); (iv) in the case of α,β-alkynylcarbonyls, the silyl group again selectively occupies the distal position of the Z-alkene (entries 10–13).11,15 For free propargylic, homopropargylic, and bishomopropargylic alcohols, hydrosilylation with a silane bearing a leaving group (e.g., an ethoxy substituent) results in the formation of a cyclic siloxane (entries 5 and 8). Significantly, the hydrosilylation using [Cp*Ru(MeCN)3]PF6 can be performed while maintaining the stereochemical integrity of asymmetric centers residing in the alkyne substrate (entry 9). Lastly, although non-sterically differentiated alkynes undergo stereo but non-regioselective hydrosilylation (entry 3), protodesilylation of the product mixture provides a single trans diastereomer.13 This provides a valuable complement to the cis-selectivity observed under Lindlar reduction conditions.
As shown in Scheme 4, even highly reactive silanes can participate in intermolecular hydrosilylation reaction, with excellent predictability.11b The resultant alkenylchlorosilane was trapped with a hexadienol to give a siloxane linkage. Heating the triene resulted in an intramolecular Diels-Alder (IMDA) reaction, yielding a siloxane with four contiguous stereocenters. The adduct could then be treated to protodesilylation or Tamao–Fleming conditions to furnish the primary alcohol or the diol respectively.
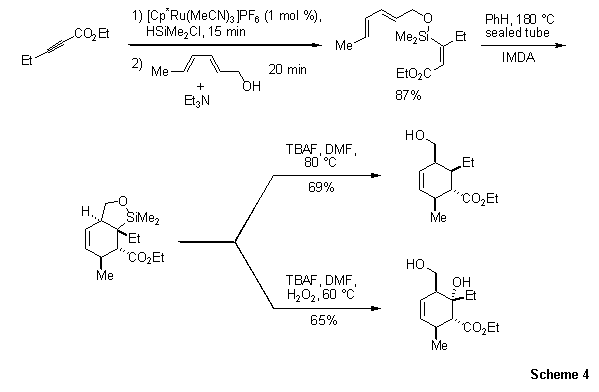
Scheme 4. Highly reactive silanes can participate in intermolecular hydrosilylation reaction, with excellent predictability.
Manipulation of the alkene prior to protodesilylation or oxidation is also feasible. For example, vinylsilanes are readily epoxidized by m-CPBA in a diastereoselective fashion (Scheme 5). Subsequent protodesilylation furnishes the corresponding syn-epoxy alcohol, while Tamao–Fleming oxidation provides a syn-diol. Therefore, this process can be used as a surrogate to the aldol condensation.
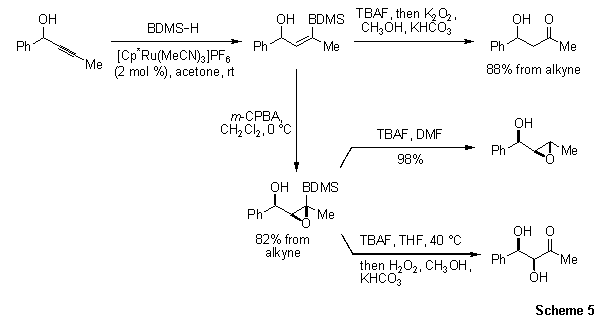
Scheme 5.Vinylsilanes are readily epoxidized by m-CPBA in a diastereoselective fashion.
Intramolecular Hydrosilylation
Finally, intramolecular hydrosilylation is possible using hydroxyalkynes, as illustrated in the concise synthesis of the
3-hydroxypiperidine alkaloid (+)-spectaline (Scheme 6).16b Treatment of the homopropargyl alcohol with tetramethyldisilazane (TMDS), followed by regio- (distal) and stereoselective (Z) intramolecular hydrosilylation, gave a cyclic azidosiloxane. Tamao–Fleming oxidation followed by reduction with concomitant cyclization gave (+)-spectaline in respectable yield.
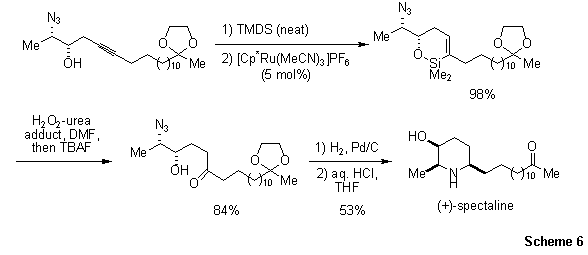
Scheme 6.Concise synthesis of the 3-hydroxypiperidine alkaloid (+)-spectaline
The sequence of intramolecular hydrosilylation and subsequent cross-coupling provides an excellent method for introducing a new carbon bond at an alkyne carbon that is in a remote position from a free hydroxyl group (Scheme 7).17

Scheme 7.Alkyne carbon that is in a remote position from a free hydroxyl group
We are pleased to offer [Cp*Ru(MeCN)3]PF6, as well as a number of other catalysts for hydrosilylation.
Materials
如要继续阅读,请登录或创建帐户。
暂无帐户?