UHPLC/MS Analysis of Cannabinoids on Ascentis® Express C18
材料
相关产品
Cannabidiol solution
1.0 mg/mL in methanol, ampule of 1 mL, certified reference material, Cerilliant®Cannabinol solution
1.0 mg/mL in methanol, ampule of 1 mL, certified reference material, Cerilliant®(−)-trans-Δ9-THC solution
1.0 mg/mL in methanol, ampule of 1 mL, certified reference material, Cerilliant®Cannabidivarin solution
1.0 mg/mL in methanol, ampule of 1 mL, certified reference material, Cerilliant®Cannabigerol solution
1.0 mg/mL in methanol, ampule of 1 mL, certified reference material, Cerilliant®Cannabigerolic acid solution
1.0 mg/mL in acetonitrile, ampule of 1 mL, certified reference material, Cerilliant®Cannabichromene solution
1.0 mg/mL in methanol, ampule of 1 mL, certified reference material, Cerilliant®Cannabidiolic acid solution
1.0 mg/mL in acetonitrile, ampule of 1 mL, certified reference material, Cerilliant®CONDITIONS
column
Ascentis Express C18, 10 cm x 2.1 mm I.D., 2.0 μm particles (50813-U)
mobile phase
[A] 0.1% formic acid; [B] 0.1% formic acid in acetonitrile
gradient
60% B to 100% B in 10 min
flow rate
0.4 mL/min
pressure
7200 psi (496 bar)
column temp.
35 °C
detector
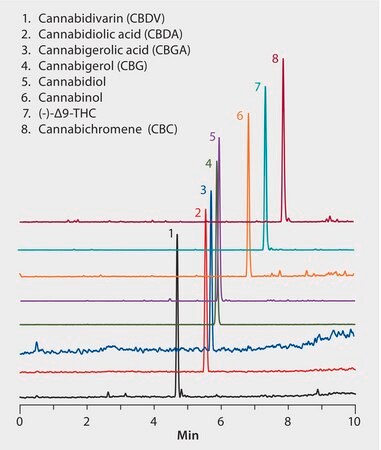
MS, ESI(+), ESI(-), MRM m/z (see figure for transitions)
injection
1 μL
sample
100 ng/mL each in methanol
说明
分析说明
Cannabis compounds reportedly have therapeutic efficacy in the treatment of pain, mood disorders, and inflammatory diseases. These standards are used in testing methods by GC/MS, LC/MS, or HPLC for applications in clinical toxicology, testing of cannabis potency or impurity profiling by growers, pharmaceutical research, forensic analysis, and urine drug testing. Shown here is the separation of cannabis compounds on an Ascentis Express C18 column. Highest grade UHPLC solvents were used to supply low background interference and low particulate contaminants for robust, trouble-free operation. Cerilliant CRMs provided reliable identification and quantification.
法律信息
Ascentis is a registered trademark of Merck KGaA, Darmstadt, Germany