Purification or Removal of Biotin and Biotinylated Biomolecules with Magnetic Beads
The magnetic beads for separation of biotinylated biomolecules have a Streptavidin ligand. Streptavidin is an Mr 60 000 protein from Streptomyces avidinii, and is a tetramer containing four biotin binding sites. Since the affinity between streptavidin and biotin is exceptionally high, harsh conditions are required for disruption, such as the use of SDS in the sample buffer. Therefore, it is also possible to use the beads for immunoprecipitation and elute binding partners in an interaction complex without coeluting the biotinylated component. The principle of immunoprecipitation is shown in Figure 1.
Streptavidin Mag Sepharose is used for efficient enrichment of biotinylated proteins, such as antibodies and immunoprecipitation. Streptavidin-coated Sera-Mag and Sera-Mag SpeedBeads are designed for isolation of biotinylated targets such as PCR products, oligos, and antibodies. The beads are generally used for increased throughput and precision in immunoassays. Neutravidin-coated and Streptavidin-blocked beads have reduced nonspecific binding, which can be beneficial in some applications.
Bead characteristics
Characteristics of Mag Sepharose, Sera-Mag, and Sera-Mag SpeedBeads for the capture of biotin and biotinylated substances are shown in Table 1.
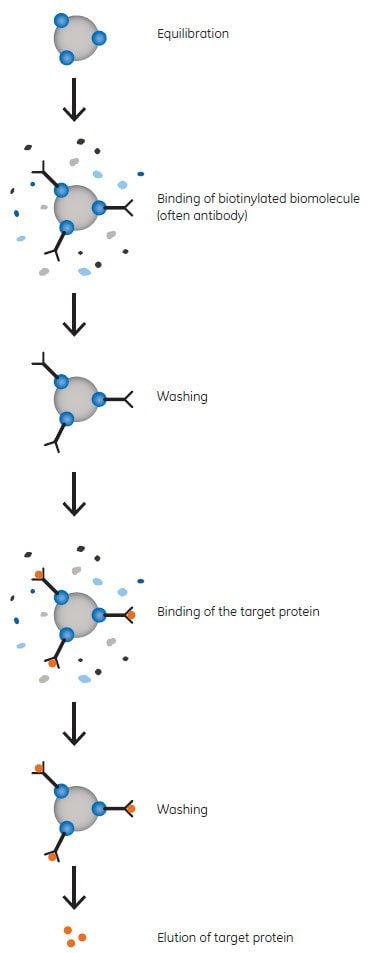
Figure 1. Principle of Immunoprecipitation
Purification Example
Immunoprecipitation of Low Concentration Transferrin from Large Sample Volumes
The ability to use different volumes of sample and medium slurry is one of the key advantages of the magnetic beads separation method. Streptavidin Mag Sepharose was used to purify human transferrin spiked in 5 mg/mL of E. coli lysate. Purification was scaled up 10-fold from 50 to 500 µl of Streptavidin Mag Sepharose and 3 to 30 mL of sample, respectively. The experiment was performed in duplicate using MagRack Maxi. The transferrin concentration was 0.75 µg/mL, which corresponds to ~ 0.015% of the total E. coli protein content. Transferrin was captured by immunoprecipitation using a biotinylated polyclonal rabbit antihuman transferrin immobilized on the medium and the yield of transferrin was estimated by SDS-PAGE of the eluted fractions.
The results (Figure 2) show the inherent flexibility of Streptavidin Mag Sepharose. A corresponding increase in the purity and recovery of transferrin was observed when the immunoprecipitation was scaled up 10-fold. The yields of transferrin were 1.2 and 13 µg using 50 and 500 µl of Streptavidin Mag Sepharose slurry, respectively. The average purity was 78% (5200-fold enrichment) and 75% (5000-fold enrichment).
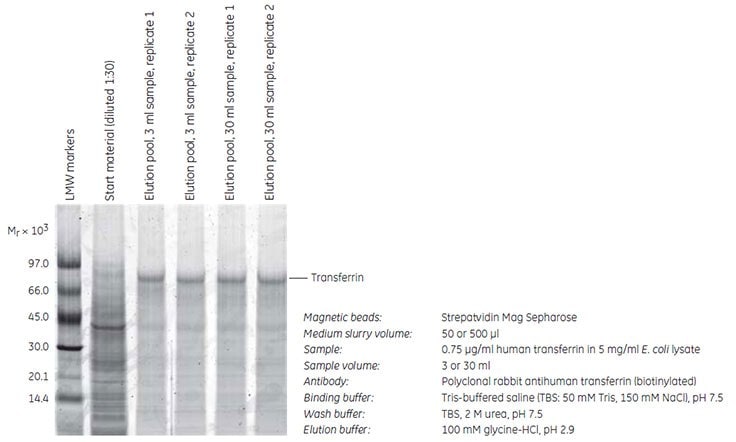
Figure 2. SDS-PAGE (reducing conditions) stained with Deep Purple Total Protein Stain. The purity and recovery obtained were equally high when the scale of purification was increased 10-fold. Quantitation of the eluted transferrin was performed using standard curves with known amounts of transferrin (data not shown).
Performing a Separation: Streptavidin Mag Sepharose
The protocols recommended below are suitable as starting points for most purifications involving biotinylated biomolecules. However, the optimal parameters depend on the specific biomolecules used and optimization may be required for best results.
Examples of parameters that might require optimization are:
- Amount of beads
- Amount of biotinylated biomolecules
- Amount of biomolecules to be enriched in immunoprecipitation
- Incubation time
- Number of washes
- Buffer compositions and pH
Sample Preparation
Adjust the sample to the composition and pH of the binding buffer. pH can be adjusted by either diluting the sample with binding buffer or by buffer exchange using PD-10 MiniTrap™ G-25 or HiTrap® Desalting columns (Appendix 1). Clarify the sample before applying it to the beads, if needed. Inhibiting protease activity in the sample prevents degradation of the target protein.
Purification of Biotinylated Proteins
Use the magnetic rack with the magnet in place to attract the beads before each liquidremoval step.
- Prepare the Mag Sepharose beads
- A. Mix the medium slurry thoroughly by vortexing. Dispense 100 µl of the homogenous medium slurry into an Eppendorf™ tube.
- B. Place the Eppendorf tube in the magnetic rack to attract the beads.
- C. Remove the storage solution.
- Equilibration
- A. Add 500 µl binding buffer and resuspend the medium.
- B. Remove the liquid.
- Apply the sample
- A. Add 300 µl of sample. If the sample volume is less than 300 µl, dilute to 300 µl with binding buffer.
- B. Resuspend the medium and incubate for 30 min with slow end-over-end mixing or by using a benchtop shaker.
- C. Remove the sample.
- Wash (perform this step three times)
- A. Add 500 µl washing buffer and resuspend the medium.
- B. Remove the liquid.
- Elute biotinylated proteins
- A. Add 100 µl elution buffer.
- B. Resuspend the medium and incubate at 95 °C to 100 °C for 5 min.
- C. Remove and collect the eluted fraction. The collected fraction contains the main part of the protein. If needed, repeat the elution.
The streptavidin-biotin bond can be broken efficiently only by harsh denaturing conditions. Hence, dissociation of biotin from streptavidin will denature both—the biotinylated protein and streptavidin, causing a leakage of the streptavidin monomer.
Immunoprecipitation
Use the magnetic rack with the magnet in place to attract the beads before each liquid removal step.
- Prepare the Mag Sepharose beads
- A. Mix the medium slurry thoroughly by vortexing. Dispense 50 µl of the homogenous medium slurry into an Eppendorf tube.
- B. Place the Eppendorf tube in the magnetic rack to attract the beads.
- C. Remove the storage solution.
- Equilibration
- A. Add 500 µl binding buffer and resuspend the medium.
- B. Remove the liquid.
- Binding of biotinylated antibody
- Add 300 µl of biotinylated antibody solution (~ 0.2 to 0.4 mg/mL). If the sample volume is less than 300 µl, dilute to 300 µl with the binding buffer.
- Resuspend the medium and incubate for 30 min with slow end-over-end mixing or by using a benchtop shaker.
- Remove the liquid.
- Wash (perform this step twice)
- A. Add 500 µl washing buffer and resuspend the medium.
- B. Remove the liquid.
- Binding of the target protein
- A. Add 300 µl of sample. If the sample volume is less than 300 µl, dilute to 300 µl with binding buffer.
- B. Resuspend the medium and incubate for 60 min with slow end-over-end mixing or by using a benchtop shaker.
- C. Remove the liquid.
- Wash (perform this step three times)
- A. Add 500 µl washing buffer and resuspend the medium.
- B. Remove the liquid.
- Elution
- A. Add 50 µl of elution buffer.
- B. Resuspend the medium and incubate for 2 min.
- C. Remove and collect the elution fraction. The collected elution fraction contains the main part of the protein. If needed, repeat the elution.
Performing a Separation: Sera-Mag SpeedBeads Streptavidin-Blocked
Sample Preparation
Combine antigen sample with 10 µg of biotinylated antibody (or biomolecule). Incubate 1 to 2 h at room temperature or overnight at 4 °C with mixing.
Dilute each sample to a minimum volume of 300 µl with cell lysis buffer or binding/wash buffer.
Immunoprecipitation
Use the magnetic rack with the magnet in place to attract the beads before each liquid removal step.
See recommended buffers for Streptavidin Mag Sepharose.
- Prepare the Sera-Mag beads
- A. Mix the medium slurry thoroughly by vortexing. Dispense 50 µl (0.5 mg) of the homogenous medium slurry into an Eppendorf tube.
- B. Place the Eppendorf tube in the magnetic rack to attract the beads.
- C. Remove the storage solution.
- Equilibration
- A. Add 1 mL of binding buffer and resuspend the medium.
- B. Remove the liquid.
- Binding of biotinylated antibody/antigen sample
- A. Add 300 µl of biotinylated antibody/antigen mixture.
- B. Resuspend the medium and incubate at room temperature for 1 h with slow end-over-end mixing or by using a bench-top shaker.
- C. Remove the liquid.
- Washing (perform this step twice)
- A. Add 300 µl of wash buffer and resuspend the medium.
- B. Remove the liquid.
- Elution
- A. Add 100 µl of elution buffer.
- B. Resuspend the medium and incubate at room temperature with mixing for 5 min.
- C. Remove and collect the elution fraction. The collected fraction contains the main part of the protein. If needed, repeat the elution.
If a low pH elution buffer is selected for elution, streptavidin may leach from the particles. Low pH elution buffers are effective for most antibody-antigen interactions. However, to ensure efficient release of target antigen from the antibody, prerinse the beads with 300 µl of 0.1% Tween 20 in water (no buffering capacity) before adding low pH elution buffer.
Alternative elution for recovery of antigen: Add 100 µl of SDS reducing sample buffer to the tube and heat the samples at 96 °C to 100 °C in a heating block for 5 min. Magnetically separate the particles and save the supernatant containing the target antigen.
Materials
如要继续阅读,请登录或创建帐户。
暂无帐户?