Inverted Organic Photovoltaic Devices Using Zinc Oxide Nanocomposites as Electron Transporting Layer Materials
Introduction
Organic photovoltaics (OPVs) represent a low-cost, lightweight, and scalable alternative to conventional solar cells. While significant progress has been made in the development of conventional bulk heterojunction cells, new approaches are required to achieve the performance and stability necessary to enable commercially successful OPVs. Inverted bulk heterojunction OPVs are one promising approach. This review highlights recent progress in high efficiency inverted polymer solar cells using zinc oxide (ZnO) as an electron-transporting layer (ETL) material, as well as new methods to improve surface and electronic properties of inverted OPVs that use ZnO.
Recent research efforts in the OPV field have largely focused on the development of novel photoactive polymers or small organic molecules for use in conventional bulk heterojunction cells. These efforts have led to power conversion efficiencies (PCEs) of up to 7–8%. In a conventional OPV cell configuration, the anode layer typically consists of a thin layer of indium tin oxide (ITO) coated with a p-type interface layer of poly(3,4-ethy lenedioxythiophene):poly(styrene sulfonate) (PEDOT:PSS). ITO is frequently used because it is conductive, transparent, and has a high work function. As a hole transporting layer, PEDOT:PSS fills pinholes in the ITO film and forms Ohmic contact with the photoactive layer. The cathode layer in a conventional OPV is typically constructed from low-work function metals such as calcium, aluminum, and magnesium. Since low-work function materials are easily oxidized when exposed to air, the cathodes must be encapsulated to prevent exposure. While some researchers have worked to develop cathodes using less reactive materials, this approach can add expense, impact performance, and/or add complexity to the OPV.
The inverted OPV configuration reverses the conventional OPV layer sequence to avoid the use of easily oxidized metals on the exposed cathode, improve device stability, and improve overall device performance. Figure 1A shows a diagram of an inverted OPV. A layer of a low-work function material is positioned directly on top of the ITO electrode to form the ETL, thus converting it to a cathode. Typical ETL materials used in inverted OPVs include cesium carbonate (Cs2CO3), n-type metal oxides such as titanium oxide (TiOx) and zinc oxide (ZnO),, as well as alcohol or water-soluble conjugated polyelectrolytes., The anode interlayer, or hole transporting layer, is most often fabricated from poly(3,4-ethylenedioxythiophene):poly(styrene sulfonate) (PEDOT:PSS) or one of many high-work function transition metal oxides including MoO3, WO3, and V2O5.4 Silver (Ag) is widely used as the exposed layer in inverted OPVs because it forms an air-stable anode and can be applied using low cost coating and printing techniques. Many materials used in inverted OPVs can be processed in solution, greatly improving prospects for future manufacturing.
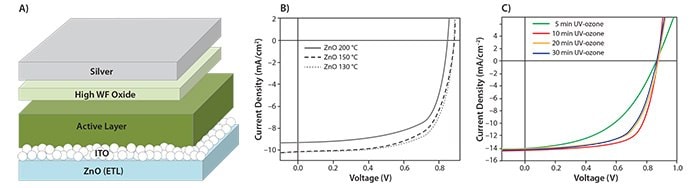
Figure 1.A) The device structure of a typical inverted polymer solar cell. B) J-V characteristics of inverted PCDTBT:PC70BM solar cells incorporating ZnO films with the indicated annealing temperatures. Adapted from Reference 13. Copyright 2011 Wiley-VCH Verlag GmbH & Co. KGaA. C) J-V characteristics of inverted PDTG–TPD:PC70BM solar cells with different UVO treatment time of ZnO-PVP ETL under 100 mWcm-2 AM 1.5 G illumination. Adapted from Reference 17. Copyright 2011 Macmillan Publishers Limited.
Low-temperature Annealed Sol-gel Derived ZnO Film as Electron Transport Layers
ZnO has shown particular promise as a cathode material for use in inverted OPV cells because of its relatively high electron mobility, stability, and transparency. In addition, ZnO thin films can be easily fabricated using highly scalable sol-gel application methods. One drawback of sol-gel methods in OPVs is the high annealing temperatures required to remove residual organic compounds and to promote oxide crystallization, which can be incompatible with polymers used in OPVs. To address this, Heeger et al.13 tested low-temperature annealing to create sol-gel derived ZnO films in OPV cells. Figure 1B shows the J-V characteristics of inverted solar cells based on poly[N-9’’-hepta-decanyl-2,7-carbazole-alt-5,5-(4’,7’-di-2-thienyl-2’,1’,3’-benzothiadiazole)] (PCDTBT, 753998) and [6,6]-phenyl C70-butyric acid methyl ester (PC70BM, 684465) that incorporate ZnO films annealed at different temperatures. These composites achieved a PCE of 6.33%. To fabricate these ZnO films, Heeger et al. applied zinc acetate in 2-methoxyethanol by spin-casting onto ITO substrates followed by thermal annealing to produce dense ZnO film by hydrolysis. While as-prepared sol-gel derived ZnO thin films are known to commonly be oxygen deficient, X-ray photoelectron spectroscopy (XPS) data from these ZnO films showed an increase of Zn-O bonds with increasing annealing temperature and thus a decrease in defect density. Additionally, the electron mobilities increased from 2.0 × 10-4 to 4.0 × 10-3 cm2 V-1 s-1 upon annealing at 200 °C. Along with high PCE, the inverted cell showed improved device stability, with stable PCE performance after exposure to air for more than 30 days. A comparable conventional cell showed a PCE drop of more than 30% after 16 hr of air exposure.
UV-ozone Treated ZnO Nanocomposites as Electron Transporting Layers
ZnO-colloidal nanoparticles have been widely used in the development of inverted OPVs due to their ease of synthesis via hydrothermal methods. However, when ZnO nanoparticles are used as the ETL in inverted OPVs, the high density of defects of the as-synthesized nanoparticles results in lower fill factors compared to conventional devices. Recent work from the So group at the University of Florida has demonstrated the performance of inverted OPVs can be improved using UV-ozone (UVO) treatment of the ZnO nanoparticles., Steady-state photoluminescence (PL) measurement of ZnO thin film showed a broad emission peaked at 519 nm, with intensity comparable to the band-to-band emission at 372 nm, indicating defects in the ZnO nanoparticles. It has been shown the defects are mainly induced by oxygen adsorption on the ZnO nanoparticle surface and such defects can be reduced significantly by UVO treatment. The reduction of defects after the UVO treatment was confirmed by large suppression of the trap emission in the ZnO thin film and prolonged carrier lifetime in OPVs. As a result, the OPVs using UVO-treated ZnO as the ETL show significant improvement in short-circuit current (JSC), suggesting the UVO treatment passivates the ZnO surface.
So et al. also developed a ZnO-poly(vinyl pyrrolidone) (PVP) nanocomposite for use as the ETLs in an inverted dithienogermolethienopyrrolodione (PDTG-TPD)-based polymer solar cell. Here, the ZnO-PVP nanocomposite replaces ZnO colloidal nanoparticles for modifying the work function of the ITO and enhancing its coupling to the active layer. Sol-gel derived ZnO-PVP nanocomposites have a number of advantages over ZnO-colloidal nanoparticles. For example, the PVP polymer passivates the ZnO surface, resulting in better stability. This passivation simplifies fabrication of the ETL device, enabling assembly in air. Second, the size and concentration of ZnO nanoclusters can be tuned by controlling the Zn2+/PVP ratio. Third, the use of PVP reduces aggregation of ZnO nanoclusters, enabling more uniform thin films. Last, PVP passivates the ZnO nanoclusters and prevents oxygen from out-diffusion and the formation of defect states, as commonly found in inorganic–organic composite thin films.
However, one drawback of the ZnO-PVP nanocomposite is the presence of PVP hinders the coupling between ZnO and the photoactive layer. As a result, devices fabricated using unmodified ZnO-PVP typically perform poorly. By treating the composite film with UVO, the coupling between ZnO and the active layer is significantly enhanced and performance improves substantially. Surface analysis of UVO-treated films reveal the PVP polymer on the film surface is effectively removed by the UVO treatment, exposing ZnO on the surface. As a result, the electronic coupling between ZnO and the active layer is enhanced, allowing more efficient charge collection in the photovoltaic device. As shown in Figure 1C, such an improvement is directly reflected on the improvement of device fill factors, which increases from below 50% to 68%. Using this ZnO composite film, inverted OPVs with PCEs over 8% can be achieved with the low bandgap polymer PDTG-TPD.
Conclusion
Despite the potential advantages of high-performance inverted OPVs, comparatively few studies have focused on their development. Significant progress has been achieved in developing inverted polymer solar cells using zinc oxide nanocomposites as electron transporting layer materials. Although there are remaining questions on how to convert the small scale lab process into the massive production of flexible solar cell films, this research opens a bright future for efficient, light weight, and low-cost OPV devices.
Materials
References
如要继续阅读,请登录或创建帐户。
暂无帐户?