Introduction to Western Blot Detection of Proteins
Introduction
The transfer of macromolecules, such as nucleic acids and proteins, to solid-phase membranous support is termed blotting. Proteins resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) are transferred to membrane composed of either nitrocellulose or polyvinylidine diflouride (PVDF) using electric current. Also referred to as protein blotting or immunoblotting, Western blotting is a powerful method for studying proteins in a sample of cells or tissue extract. The method involves five main steps detailed below.
Sample preparation
Preparing the sample includes protein extraction from cells or tissues and estimation of protein concentration. Various lysis buffers containing detergents and protease and phosphatase inhibitors may be used to solubilize proteins from whole tissue or tissue culture extracts. The total protein in the samples may be quantified spectrophotometrically at 595 nm using Bradford reagent.
Gel electrophoresis
Proteins may be separated based on isoelectric point, molecular weight, electric charge or a combination of all these. The most popular method is electrophoresis using polyacrylamide gels loaded with SDS (SDS-PAGE). The protein samples are denatured by boiling with a reducing agent to break the disulfide bonds before electrophoresis.
Transfer of proteins
The proteins separated by gel electrophoresis are immobilized by transfering to a solid support, such as PVDF or nitrocellulose membrane. The transfer uses electric current to pull proteins from the gel onto the membrane (electroblotting). View the SNAP i.d.® 2.0 Blot Roller and other accessories.
Validation of proteins
For detection of a target protein transferred onto the membrane, it is important to block the detection of any non-specific proteins that results in false-positive results. This is usually done by using blocking agents such as BSA, non-fat milk or specific antiserum. Thereafter, the membrane is incubated with optimized concentrations of primary and secondary antibodies specific to the target protein.
Detection and visualization
Proteins of interest may be detected by colorimetric, radioactive, chemiluminescent or fluorescent methods. The secondary antibody is typically conjugated to horseradish peroxidase that cleaves a chemiluminescent agent. The luminescence produced by the reaction product is proportional to the amount of the target protein.

Figure 1:Sigma-Aldrich Blotting and Vertical Electrophoresis System
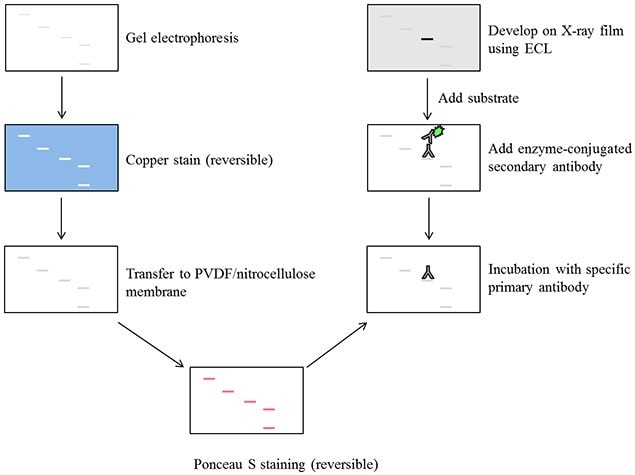
Figure 2. Steps involved in western blotting procedure
Sample preparation
Extraction of proteins
All the steps for protein extraction from cells or tissue (fresh or frozen) must be performed at 2-8 °C. The following is the composition of a common lysis buffer used to prepare protein samples.
RIPA Buffer:
- NaCl (S3014) 150 mM
- Triton X-100 (T8787) 1%
- 0.5% Sodium deoxycholate
- 0.1% SDS (L3771)
- 50 mM Tris-HCl pH 8.0
- Protease inhibitors
Extraction of proteins from adherent cells
- Discard the medium in culture dishes with cells and wash the cells using ice-cold PBS.
- Discard the PBS, add ice-cold lysis buffer.
- Scrap the cells using cold plastic cell scraper. Collect the cells in microcentrifuge tubes.
- Agitate the contents in microcentrifuge tubes for 30 min at 4 °C.
- Centrifuge the tubes at 16,000 x g for 20 min at 4 °C. Collect the supernatant in fresh tube and place on ice. Discard the pellet.
Extraction of proteins from cells in suspension
- Centrifuge the cell suspension at 2,000 x g for 5-7 min at 4 °C. The cells are collected at the bottom of the tube, discard the supernatant.
- To the cell pellet, add ice-cold PBS and wash the cells by centrifuging at 2,000 x g for 5-7 min at 4 °C.
- Add ice-cold lysis buffer to the cell pellet. Agitate the contents in microcentrifuge tubes for 30 min at 4 °C.
- Centrifuge the tubes at 16,000 x g for 20 min at 4 °C. Collect the supernatant in fresh tube and place on ice. Discard the pellet.
Extraction of proteins from tissues
- Dissect the tissue of interest on ice. Transfer the tissue to round-bottomed microcentrifuge tubes and snap-freeze by immersing in liquid nitrogen.
- For 5 mg tissue, add 300 µL of ice-cold lysis buffer and homogenize using electric homogenizer. Add additional 300-600 µL of lysis buffer during homogenization.
- Agitate the contents for 2 h at 4 °C.
- Centrifuge the tubes at 16,000 x g for 20 min at 4 °C. Collect the supernatant in fresh tube and place on ice. Discard the pellet.
Protein estimation
- Take a small volume of lysate to perform protein estimation assay. Protein estimation may be performed using Coomassie protein assay reagent (Product No. 27813).
- Record the absorbance of the standards and unknown samples at 595 nm.
- Determine the protein concentration of unknown samples by comparison with the standards.
- Transfer appropriate volume of lysates to microcentrifuge tubes so that all samples will content the same total protein concentration.
- Add adequate ice-cold lysis buffer to make up all the lysates to the same volume.
Sample preparation
The following is the composition of loading buffer required to prepare the samples for electrophoresis.
2X Laemmli loading buffer:
- Bromophenol blue (B5525) 0.004%
- 2-mercaptoethanol 10%
- Glycerol (G5516) 20%
- SDS (L3771) 4%
- Tris-HCl 0.125 M
- To a volume of cell lysate, add equal volume of loading buffer.
- Boil the above mixture at 95 °C for 5 mins. Centrifuge at 16,000 x g for 5 mins.
- These samples can be stored at -20 °C or may be used to proceed with gel electrophoresis.
Gel Electrophoresis
Running buffer that acts as both anode and cathode buffer is available (Product No. GE28-9902-52). Alternatively, running buffer can be prepared from individual components.
- 10X gel running buffer, pH ~8.6
- Tris 250 mM
- Glycine (G8898) 1.92 M
- SDS (L3771) 1%
- Load equal amounts of proteins into discontinuous precast gels, with stacking gel component and a resolving gel component.
- Include a protein marker, if required.
- Run the gel at 80-100 V for stacking gel and once the samples enter the resolving gel increase the voltage to 100-150 V, till the bromophenol blue dye front reaches the end of the gel.
Gel staining
R-PROB staining
- Immerse the gels post-electrophoresis in fixing solution for 20 mins. Repeat this step twice.
- Rinse the gel in water twice, each wash lasting 30 mins.
- Incubate the gel in Reversible Protein Detection Kit for Membranes and Polyacrylamide Gels (Product No. RPROB) for 20-40 mins with gentle agitation.
- Wash excess stain in 10% acetic acid.
- For destaining, wash the gel with EDTA followed by two washes of 15 mins each in water or fixing solution.
Copper staining
To confirm the migration and separation of proteins, the gel may be stained with a reversible stain such as CuCl2.
- Rinse the gels post-electrophoresis in distilled water for a maximum of 30 min.
- Immerse the gel in 0.3 M CuCl2 solution for 10 min. Briefly, rinse with de-ionized water.
- The proteins can be visualized as clear zones in a blue background. Photograph the gel if required.
- For destaining, wash the stained gels in 0.25 M Tris and 0.25 M EDTA solution, pH 9, repeatedly.
- Move the destained gel to transfer buffer before proceeding with the transfer setup.
Transfer of proteins
The transfer of proteins from the gel to a flexible and easily-handled membrane support can be performed rapidly using electricity, a process called as electroblotting. Electroblotting can be performed in wet or semi-dry conditions. In both procedures, the main components required are the transfer buffer and the transfer unit.
Reagents
- 1X transfer buffer (prepared from 10X transfer buffer, Product No. T4904)
- Methanol (Product No. M1775)
- PVDF (Product No. 05317) or nitrocellulose (Product No. GE1060000) membrane
Before preparing the transfer setup, the following equilibration steps are required for PVDF membranes:
- Cut an appropriate size of PVDF membrane.
- Soak the membrane in methanol for 2 min.
- Remove methanol and incubate the membrane in cold transfer buffer for 5 min.
- The PVDF membrane is now equilibrated to be used for transfer of proteins.
DO NOT incubate nitrocellulose membranes in methanol. Incubation in transfer buffer is sufficient.
To transfer the proteins separated from the gel to the membrane, the following stack has to be prepared in blotter unit. Ensure no air bubbles are trapped between the gel and the membrane.

Figure 3.Western blot transfer assembly
We offer semi-dry blotter units that conserve buffer volume, produce less heat, less band distortion and allows for the transfer of multiple gels of varying thickness.
The transfer can be performed at a voltage of 50 V at 4 °C for 2 h.
Reversible staining of membranes for protein detection
The transfer of proteins from gel to membrane may be confirmed by using a reversible membrane stain such as Ponceau S. The following are the reagents required for reversible staining of the membrane.
- Ponceau S solution (P7170)
- TBST buffer: There are several types of TBST buffer:
1. 1X TBST (T9039)
2. 10X TBS (T5912); add 0.05% TWEEN® 20 (P9416) before use
3. Prepare using the following reagents: NaCl (S3014) 0.15 M, Tris-HCl 0.05 M, TWEEN® 20 (P9416) 0.05% - NaOH 0.1 M
Procedure
- Wash the membrane in TBST (if the membrane has been air dried after transfer, the membrane should be immersed in TBST till it is completely wet again).
- Immerse the membrane in the stain solution for 5 min. The proteins can be visualized as red/pink bands on the membrane.
- For destaining, immerse the membrane in 0.1 M NaOH; the protein bands begin to disappear after ~30 sec.
- Rinse the membrane in running distilled water for 2-3 min.
- The completely destained membrane is now suitable for protein validation using antibodies.
Protein validation
Blocking solutions
We offer prepared blocking solutions, such as Product No. W0138, T8793, B6429 and C7594. Non-fat, dry 3% milk in TBST buffer OR 5% BSA (A7906) dissolved in TBST buffer can also be used.
- If blocking solution is being prepared, filter the solution to avoid dark grainy background on the blot during development. The blocking solution with BSA is preferable for the detection of phosphorylated proteins.
- Block the membrane in blocking solution for 1 h at 4 °C. For best results, optimize the blocking time.
- Wash the membrane in TBST for 1 min after incubation with blocking solution.
Incubation with primary antibody
- Dilute the primary antibody in TBST as recommended in the datasheet. Some antibodies are diluted in 5% BSA in TBST solution.
- Incubate the membrane in primary antibody solution for few hours to overnight at 4 °C. Incubation of membrane in antibody solution with rocking motion is recommended as it facilitates even binding.
- After incubation, remove the primary antibody solution. Wash the membrane with TBST in three washes of 5 min each.
Incubation with secondary antibody
Commonly used secondary antibodies are conjugated with horseradish peroxidase (HRP) or alkaline phosphatase (ALP). HRP-conjugated secondary antibodies are most sensitive compared with the latter.
- Dilute the secondary antibody in TBST as recommended in the instructions.
- Incubate the membrane in secondary antibody solution for 1-2 h at room temperature with agitation.
- After incubation, remove the secondary antibody solution. Wash the membrane with TBST in three washes of 5 min each.
Detection
Various methods for protein detection on Western blots are available, including colorimetric and chemiluminescent reagents for horseradish peroxidase and alkaline phosphatase.
See a list of recipe calculators for various Western blotting buffers and blocking solutions.
Materials
References
如要继续阅读,请登录或创建帐户。
暂无帐户?