LC-MS/MS Analysis of 40 Per- and Polyfluoroalkyl Substances (PFAS) in Solvents: A Modified EPA Method 1633A Approach
Abstract
A modified workflow based on EPA Method 1633A was applied for the analysis of 40 per- and polyfluoroalkyl substances (PFAS) in a range of solvents. Solid-phase extraction (SPE) combined with LC-MS/MS was used to assess the PFAS background of 19 solvent products, including water, acetonitrile, and methanol of different grades. Ensuring solvent purity was identified as essential for achieving reliable PFAS measurements and for preventing false positives or overestimation of PFAS concentrations.
Section Overview
Introduction
Per- and polyfluorinated alkyl substances (PFAS) are a class of synthetic compounds that have been widely used in consumer and commercial products for several decades because of their versatile physical and chemical properties (e.g., in water repellents, firefighting foams, cookware, and food packaging). Due to their chemical stability arising from the strong carbon-fluorine bond, their persistence, and their toxicity, these compounds have gained increasing attention with respect to contamination of water, soil, and food, along with the associated bioaccumulation in humans and animals.
Guidance for PFAS testing regulations has been issued by the United States Environmental Protection Agency (US EPA) and the European Union (EU) to support the protection of environmental and human health, including EPA methods 533, 537.1, and 1633A and FDA method C-010.03.1-6
In December 2024, the US EPA released the final version of EPA Method 1633A, which provides extensive guidance for environmental PFAS testing. The method was validated across multiple laboratories and includes the analysis of 40 PFAS compounds in a wide range of sample matrices, including aqueous samples such as wastewater, surface water, and groundwater, solid samples such as soil, sediment, and biosolids, and biological samples such as fish and other tissues. For PFAS analysis in food, advisories were issued by the U.S. Food and Drug Administration (FDA) by releasing the method C-010.03, which employs a modified QuEChERS (Quick, Easy, Cheap, Effective, Rugged, and Safe) procedure followed by dispersive solid phase extraction (dSPE) for clean-up before liquid chromatography-mass spectrometry (LC-MS) detection.
The analysis of PFAS can be performed using the analytical methods described in the guidance documents by following their established matrix-specific extraction protocols for detecting these contaminants in various sample matrices. A key requirement for the successful application of these methods is the high purity of the solvents used, such as water, acetonitrile, and methanol, which are required during both the analytical and preparative stages of the analysis, such as the preparation of the mobile phase, extraction reagent, and calibration standards. The high sensitivity of LC-MS instruments and the need for trace-level PFAS detection necessitate the use of solvents that are free of any PFAS contamination. Any PFAS present in these solvents can lead to misleading results in the form of false positives or overestimation of PFAS levels, which compromises the validity of the analytical results and the conclusions. Ensuring solvent purity is therefore essential for achieving accurate and reliable PFAS analysis.
This study describes the analysis of 40 PFAS analytes in bottled water, acetonitrile, and methanol from two portfolio brands (Supelco® and Sigma-Aldrich®) across various solvent grades, including HPLC grade, HPLC plus grade, gradient grade, UHPLC grade, and LC-MS grade. Solid-phase extraction (SPE) was performed using Supelclean™ ENVI-WAX™ SPE cartridges in combination with a PTFE-free Visiprep™ SPE vacuum manifold. The extracts were subsequently analyzed by LC-MS/MS using analytical and delay Fused-Core® Ascentis® Express PFAS columns.
Experimental
Solutions and Standards Preparation
40 native and 31 isotopically labeled PFAS standards were used as methanolic 50 µg/mL stock solutions. Following the recommendations of the EPA 1633A method, these native and isotopically labeled standards were then diluted to prepare seven calibration solutions (CS1 - CS7). The calibration solutions contained the native PFAS compounds at concentrations ranging from 0.2 to 5 ng/mL for CS1 and from 62.5 to 1560 ng/mL for CS7, as specified in Table 4 of EPA Method 1633A, to establish the working range of the MS instrument.
Sample Preparation for Solvent testing
Sample collection and preparation were performed according to EPA Method 1633A with slight modifications. Three solvents, namely water, acetonitrile, and methanol (1 L bottle size), were examined for potential PFAS contamination across the grades listed in Tables 3 to 5. Volumes of 500 mL of water, 250 mL of acetonitrile, or 250 mL of methanol were transferred into HDPE bottles fitted with linerless polypropylene caps. To the acetonitrile and methanol samples, 500 mL or 250 mL of UHPLC grade water (1.03728) were added, respectively, to dilute the organic solvents and prevent poor recovery of selected PFAS compounds due to insufficient retention during the SPE loading step. The samples were fortified with 24 isotopically labeled standards (13C or D), which served as extracted internal standards (EIS).
For SPE, Supelclean™ ENVI-WAX™ SPE tubes (500 mg/6 mL, 54057-U) were fitted with large volume SPE reservoirs (25 mL, 54258-U) and placed on a PTFE-free Visiprep™ vacuum manifold (57030-U). The tubes were conditioned with 15 mL of 1.0% NH4OH in methanol and equilibrated with 5 mL of aqueous 0.3 M formic acid. The samples (500 mL of water, 500 mL of diluted methanol or 750 mL of diluted acetonitrile as described above) were then loaded and passed through the cartridges. The washing step consisted of two rinses with 5 mL of water followed by 5 mL of 0.1 M formic acid in water/methanol (1:1, v/v). The cartridges were subsequently dried for 1 min before elution of the analytes with 5 mL of 1.0% NH4OH in methanol into collection tubes that contained seven additional isotopically labeled standards. These standards, used as non-extracted internal standards (NIS), were included to evaluate the recovery of the EIS compounds and assess the applicability of the method. Prior to LC-MS/MS analysis described below, 25 µL of concentrated acetic acid (5.33001) were added to the eluates.
Instrumental Analysis
LC-MS/MS analysis was performed using an Agilent 1290 Infinity II instrument coupled to an Agilent 6495C triple quadrupole mass spectrometer. Analyte separation was achieved on an Ascentis® Express PFAS 90 Å column (5 cm x 2.1 mm, 2.7 µm, 53557-U) used as the analytical column. An Ascentis® Express PFAS Delay 160 Å column (5 cm x 3.0 mm, 2.7µm, 53572-U) was installed after the mixing valve and before the autosampler to offset PFAS contamination potentially originating from the LC system (e.g., pump, tubing, fittings, filters). Polypropylene snap cap vials were used instead of standard glass vials to avoid possible adsorption of PFAS to glass surface. The LC-MS employed for the analysis are summarized in Tables 1 and 2, including the used MRM transitions for quantification of the native PFAS by stable-isotope dilution. The allocation of extracted internal standards followed the specifications of EPA Method 1633A.
Table 2. MRM (ESI-) transitions, chromatographic and linearity data for the 40 native PFAS
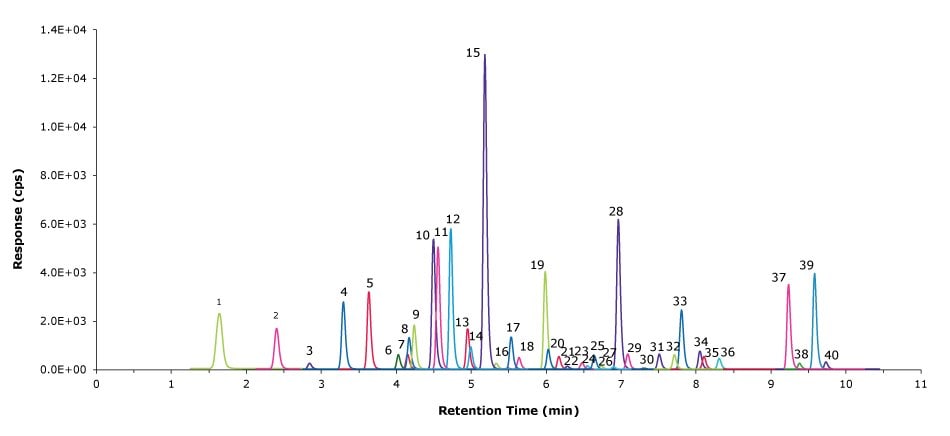
Figure 1.LC-MS/MS chromatogram of 40 PFAS compounds obtained for CS5 in methanol containing 4% water, 1% ammonium hydroxide, and 0.6% acetic acid (Peak IDs see Table 2).
Potential PFAS contamination in the solvents was quantified by stable-isotope dilution using extracted internal standards (EIS) that were added to the sample (n = 2) prior to SPE. Recovery of the EIS surrogates was assessed using the non-extracted internal standards (NIS), which were spiked into the concentrated extract after the SPE step. The PFAS evaluation of the three solvents across the various grades revealed negligible levels of all investigated PFAS compounds, as all measured values were below the respective lower limits of quantification (LOQ) specified in EPA method 1633A (Table 3) for each of the compounds. To account for the varying analyzed sample volumes (500 mL of water, 250 mL of pure methanol, and 250 mL of pure acetonitrile) which result in different LOQs for each solvent, the LOQ for each PFAS compound was additionally calculated for a 1 L bottle size and expressed in parts per trillion (ppt) to improve comparability of the data (Table 4).
Tables 5, 6, and 7 present the recoveries determined for the 24 EIS surrogates in the investigated solvents, calculated using the seven NIS compounds that were spiked after extraction. The EPA requirements for acceptable recoveries were met for all analytes examined, as outlined in Table 6 of EPA Method 1633A1, with most compounds exhibiting recoveries between 73.4% and 109.8%. Lower recoveries between 20.7% and 39.0% were observed for d7-NMeFOSE and d9-NEtFOSE in acetonitrile, which may be attributed to insufficient retention during the SPE loading step caused by the higher eluotropic strength of the acetonitrile samples despite prior dilution.
Table 4. Compound-specific LOQ values of EPA method 1633A expressed in ng/mL and corresponding LOQ values for the analyzed solvent samples expressed in ppt
Perfluoroalkyl carboxylic acids (LOQ: 0.2 – 0.8 ng/mL)
Perfluoroalkyl sulfonic acids (LOQ: 0.2 ng/mL)
Fluorotelomer sulfonic acids (LOQ: 0.8 ng/mL)
Perfluorooctane sulfonamides (LOQ: 0.2 ng/mL)
Perfluorooctane sulfonamidoacetic acids (LOQ: 0.2 ng/mL)
Perfluorooctane sulfonamide ethanols (LOQ: 2 ng/mL)
Per- and Polyfluoroether carboxylic acids (LOQ: 0.4 - 0.8 ng/mL)
Ether sulfonic acids (LOQ: 0.4 - 0.8 ng/mL)
Fluorotelomer carboxylic acids (LOQ: 1.0 - 5.0 ng/mL)
Table 5. Recoveries (n = 2) of Extracted Internal Standards (EIS) for the tested water solvents, together with the acceptance criteria specified for aqueous matrices in EPA method 1633A
Table 6. Recoveries (n = 2) of Extracted Internal Standards (EIS) for the tested acetonitrile solvents, together with the acceptance criteria specified for aqueous matrices in EPA method 1633A
Table 7. Recovery (n = 2) of Extracted Internal Standards (EIS) for tested methanol solvents, together with the acceptance criteria specified for aqueous matrices in EPA method 1633A
Conclusion
An adjusted workflow based on EPA method 1633A was applied for the analysis of 40 PFAS compounds in water, methanol, and acetonitrile of various grades using SPE with a Supelclean™ ENVI-WAX™ cartridg,e followed by LC-MS/MS quantification with Ascentis® Express PFAS analytical and delay columns. Robust method performance was demonstrated by satisfactory recovery values for all 24 EIS surrogates, which fell within the acceptance range of EPA method 1633A. None of the investigated solvents showed PFAS contamination at levels exceeding the respective LOQs defined in EPA Method 1633A, indicating their suitability for trace-level PFAS analysis according to the relevant analytical methods issued by regulatory agencies. Laboratories operating under the EPA 1633 guideline remain responsible for independent verification of each reagent lot to avoid issues with method blanks and other quality control samples. When available, certification of PFAS levels in additives and solvents provided by the supplier is sufficient for meeting this requirement.
Enhance your PFAS analysis with high-quality standards and sample preparation solutions designed for reliable, trace-level detection. Explore the full portfolio and find the right tools for your workflow at SigmaAldrich.com/PFAS.
See also our recently introduced solvents tested for PFAS analysis.
Solvents
Reagents
Certified Reference Materials
References
如要继续阅读,请登录或创建帐户。
暂无帐户?