Substituted Adamantyl Phosphinoferrocenes (AdQPhos) and Precatalysts for Sustainable α-Arylation Reaction in Water
Abstract
Novel polyarylated admantylphosphino ferrocene (AdQPhos) and its Pd-precatalysts were synthesized in multi-gram quantities in excellent yields and purity. This catalytic system demonstrated remarkable activity and selectivity towards palladium-catalyzed α-arylation reactions, accommodating a wide range of nucleophiles, including nitroalkanes, nitriles, amides, esters, and ketones. Compared to the other state-of-the-art catalysts, this system exhibited superior performance in all these aforementioned transformations. Furthermore, by integrating micellar catalysis, these catalysts enabled such carbanion-mediated C-C couplings under mild aqueous conditions, mitigating the need for hazardous solvents like 1,4-dioxane (D201863) or NMP (M79603). Notably, this work also presents the first report for the α-arylation reactions involving cyclic amides under aqueous conditions. Interestingly, the α-arylations of esters proceeded smoothly without employing Zn-enolates (Referomosky reagents), addressing the step-efficiency challenges associated with these reactions. In addition, challenging α-arylations of 3o enolates as nucleophiles to synthesize 4o carbon centers were also demonstrated with the AdQPhos-based catalytic system. A pentagram scale example demonstrated the scalability of this reaction.
Section Overview
Introduction
Palladium-catalyzed α-arylation reaction has emerged as an important class of reactions primarily due to their ability to forge C-C bonds between heavily functionalized molecules.1–8 Such transformations are extremely relevant in the synthesis of APIs,2,3 natural products,4,5 and agrochemicals.6 Mechanistically, these reactions proceed through the traditional Pd(0)/Pd(II) cycle, where, the transmetalating nucleophile is typically derived from an enolate.9 This approach gives a facile and convenient route to access α-arylated carbonyl compounds, compared to the traditional multi-step cyclization strategies or SNAr reactions carried out under harsher conditions.10,11 The first generation variants of these reactions either employed preformed enolates12,13 or Zn-reagents (Reformatsky reagent)14 as the nucleophiles. This approach was extremely step-inefficient and raised considerable operational difficulties, especially during the scale-up. To overcome some of these challenges, independent work from Buchwald15 and Hartwig16 showcased the utilization of in situ generated enolates from the respective carbonyl compounds. Since then, the field has expanded significantly with the respect to the range of enolate precursors, encompassing aldehydes,17 amides,18 esters,18–20 acids,21 acid chlorides,22 nitroalkanes,23 nitriles,24 and more.9 Process chemists have explored this technology at scale for the synthesis of various APIs and agrochemicals.2,3
Despite significant progress in Pd-catalyzed α-arylation of carbonyl compounds, several key challenges remain (Figure 1). A major limitation is the lack of a general and versatile ligand/catalytic system capable of efficiently accommodating a broad range of nucleophiles. This substantially impacts the overall process efficiency, as chemists must dedicate a significant amount of time and resources into identifying an optimal ligand/catalytic system for each desired transformation. Additionally, α-arylations involving tertiary-carbon centered nucleophiles remain poorly accommodated in this class.23 This is primarily due to the inability of the traditional catalytic systems to facilitate the transmetallation of the sterically challenging nucleophiles or the reductive elimination of coupling partners from the Pd-center.24,25 As a result, these reactions often require elevated temperatures or sophisticated ligands/precatalysts to proceed.26 In addition to substrate and catalyst limitations, scalability remains a major concern, with many reported protocols proving difficult to reproduce under preparative or industrial conditions. Moreover, the prevailing reaction conditions rely heavily on using anhydrous hazardous organic solvents, such as dioxane, NMP, and benzene. The resulting environmental and safety issues, compounded by increasingly stringent global regulations, adversely affect the widespread adoption of this methodology for bulk chemical production.
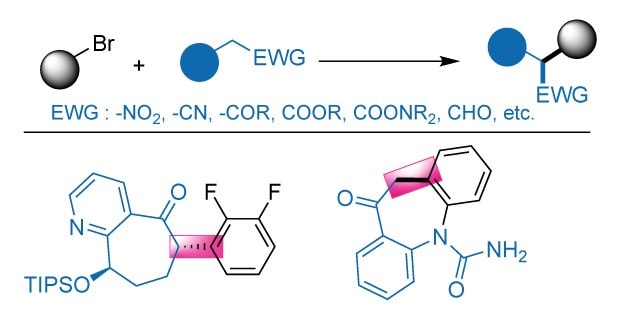
Figure 1.Pd-Catalyzed α-arylation reactions.
Notably, modern sustainable chemistry practices have identified these hazardous solvents as the major contributor to the overall waste generation and pollution. From a pharmaceutical industry perspective, about 85% of the mass employed for an API synthesis is associated with solvent consumption.27 In addition, many of these solvents also pose substantial health risks to workers.28 For instance, NMP (M79603) and DMF (D158550) are reported to be highly toxic to the human reproductive system.28 Due to these reasons, various environmental agencies are bringing stronger regulatory legislations to limit their use unless it is highly necessary. This forces chemists to find greener alternatives.29,30 One prevailing approach in this field is to employ water as the bulk reaction medium by following mother nature’s lead.31,32 Over the last two decades progress has been made in this area by employing water as the bulk reaction medium, primarily enabled by micellar catalysis.33,34 These micelles act as the “nanoreactors” in the bulk medium, facilitating a multitude of synthetic transformations under milder aqueous conditions.33,35 The pioneering contributions by Lipshutz,36,37 Handa,38,39 Gallou,40,41 Kobayashi,42 and others43,44 have substantially advanced the field. Increasing adoption of this technology by agrochemical and pharmaceutical industries reflects its importance in the context of sustainability and green chemistry.45,46
Hence, our goal is to leverage the use of water as a solvent for palladium-catalyzed α-arylations under milder conditions to improve sustainability and safety. Conceptually, employing water as the reaction medium is not ideal for these transformations as the carbanion or related similar intermediates are unstable under aqueous conditions. Recent independent reports from the groups of Handa and Lipshutz demonstrated that the “shielding effect” of micelles can extend the life span of these sensitive intermediates under aqueous conditions and effectively enable the desired transformations.47–50 This was demonstrated in the α-arylations of nitroalkanes,49 phenyl acetonitriles,47 and ketones.50 Careful evaluation of these reports revealed the exhaustive and time consuming screening studies were performed in each of these α-arylations to identify the ideal ligand/catalytic systems. For instance, while the cationic complex t-BuXPhosPd(crotyl)OTf, developed by Colacot et.al.,51 was demonstrated as the most effective catalyst in α-arylations of nitroalkanes, XPhosPd(crotyl)Cl developed by the same group, was used as a “nanocatalyst precursor for α-arylations of nitriles,51 and the highly air sensitive [PdP(t-Bu)3Br]2 was concluded as the most active catalyst in α-arylations of ketones.50 These examples demonstrate the first challenge discussed earlier: “unavailability of a versatile ligand/catalyst system for these reactions.” Therefore, it is an important need to develop a general ligand capable of accommodating a wide range of nucleophiles for all classes of α-arylations under milder conditions, although we are aware that there is not a universal catalyst for every system. However, such a leap could add considerable value to synthetic chemists not only in academia but also for those who want to explore this technology for real-world applications.
A comprehensive literature evaluation in this direction unveiled the progression of ligands employed in these reactions, ranging from the bidentate BINAP (481084) and XantPhos (526460) to the more advanced Buchwald's XPhos (638064) and RuPhos (663131),52,53 as well as Hartwig's QPhos (675784) ligands.54,55 Among these, the substitution patterns on Hartwig's QPhos are notable, as such a polyarylated phosphino-ferrocene exhibited excellent activity toward a broad range of cross-coupling reactions.
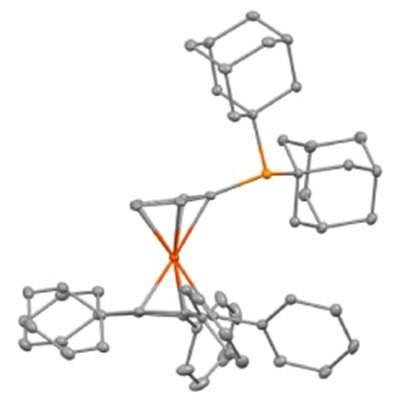
Figure 2.Thermal ellipsoid plot of the AdQPhos ligand.
Given our work in developing ferrocene-based ligands, we decided to develop a new catalyst using the polyarylated ferrocene core.56 Modern ligand designs have demonstrated that the incorporation of bulky adamantyl groups on the phosphine has resulted in remarkable enhancement of catalytic properties, as evidenced by Buchwald's findings with AdBrettPhos,57 Beller's studies on CatacXium,58 Stradiatto's research on MorDalphos derivatives,59 Carrow's work on Ad3P60 and our recent report on MPhos.56 By combining adamantyl technology with the uniqueness of the QPhos Cp core, we envisioned that adamantyl phosphino ferrocenes (AdQPhos) might exhibit some unique activities in the area of cross-coupling (Figure 3). Moreover, we sought to establish a robust and scalable synthetic protocol to enable the broader application of this ligand class in preparative and industrially relevant cross-coupling processes.
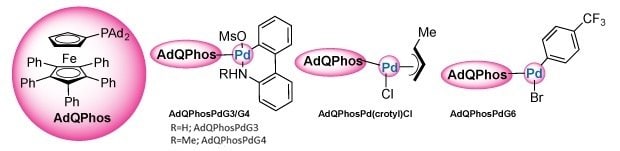
Figure 3.AdQPhos and its precatalysts.
Herein, we describe a very convenient and scalable synthesis of a new class of adamantyl phosphino ferrocene (AdQPhos 936189) and its palladium complexes. These compounds exhibited remarkable catalytic activity in various kinds of challenging α-arylation reactions accommodating a broad range of nucleophiles. Studies were conducted in organic solvents and in green aqueous conditions with relatively low Pd loadings. We also explored these precatalysts for the challenging α-arylations of tertiary centers to test their ability to create quaternary centers. The results of the study are summarized below.
Result and Discussion
Phosphine Ligand synthesis
The ligand synthesis started with the lithiation of readily available starting material, bromoferrocene (725242), followed by quenching with Ad2PCl (737267) (Scheme 1, Part (a)). Interestingly, the ligand (1b) was isolated by simple filtration as the product crashed out from the reaction mixture. The corresponding tBu2P ligand synthesis is extremely tedious due to the isolation problems associated with the instability of the intermediate in the solution phase, byproduct formation, and, as a consequence, the purification process typically requires chromatography.54,55 From a process chemistry perspective, our approach is much more process-economical and convenient. Notably, the traditional installation of Ad2P group requires harsh palladium-catalyzed coupling conditions56 or requires stoichiometric or catalytic amounts of copper salt, especially while synthesizing sterically hindered phosphines, i.e., AdBrettPhos (768154) or MorDalPhos (751618).57,59 Further, adopting a similar procedure used for QPhos synthesis,54,55 the penta-arylation of the bottom cp ring of 1a was achieved cleanly under palladium-catalyzed conditions to form the desired product 1b.
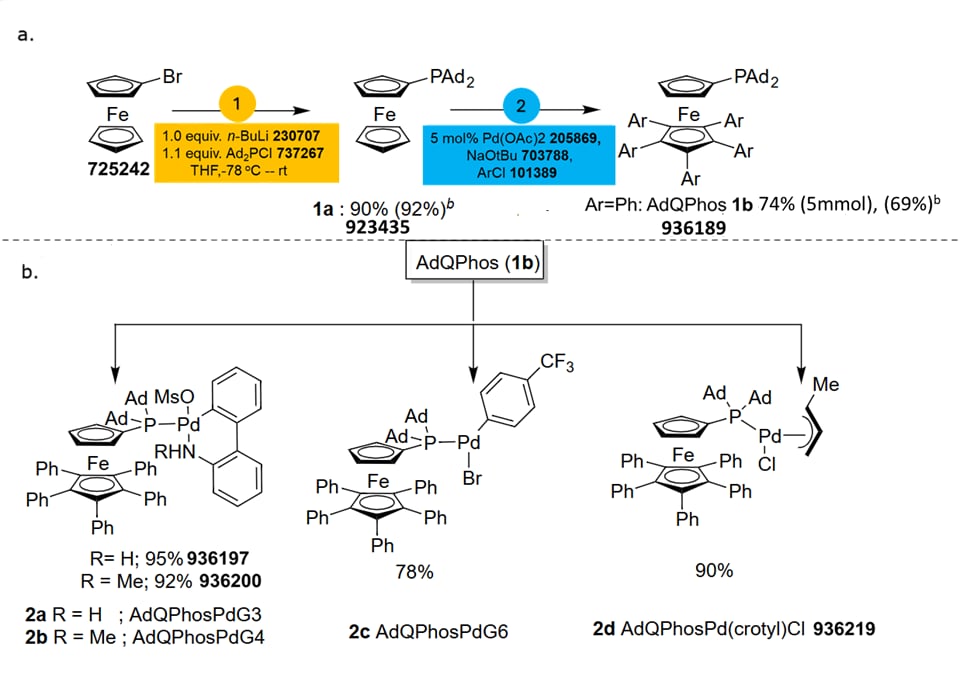
Scheme 1.Synthesis of (a) AdQPhos and (b) AdQPhos precatalysts.
aConditions: bromoferrrocene (5.0 mmol), n-BuLi (5 mmol), Ad2PCl (5.5 mmol), THF (55 mL), -78 - 23 °C, 19 h; 1a (4.45 mmol), NaO-tBu (44.5 mmol), ArCl (445 mmol), Pd(OAc)2 ( 0.225 mmol), 110 °C.
breaction scale was at 30 g of starting material.
Unlike the QPhos synthesis, which typically requires 10 mol% Pd loading,54,55 the synthesis of our AdQPhos required only half of the Pd loading (5 mol%), although the process was not fully optimized. We would also like to emphasize that the synthetic protocol of AdQPhos does not rely on tedious work-up or purification techniques such as chromatography in comparison to QPhos. In the case of our AdQPhos, the isolation required only simple filtration followed by washing to get analytically pure material. We were also able to scale up the process to 30 g with similar isolated yield in excellent purity. Subsequently, the Pd complexes such as Buchwald's palladacycles G3 936197 (2a), G4 936200 (2b), oxidative addition complex (G6 2c), and Colacot's crotyl 936219 (2d) were synthesized following the literature procedures in good to excellent yields (Scheme 1, Part (b)).51,61 All the ligands and precatalysts were fully characterized by NMR spectrometry, mass spectroscopy, and other analytical methods. The authenticity of the ligand AdQPhos (1b) was further confirmed with a single-crystal X-ray study (Figure 2).
Application Studies
With AdQPhos and its precatalysts in hand, we initiated our application studies focusing on assessing their efficacy in various α-arylations encompassing challenging nucleophiles, including enolates derived from amides, esters, ketones, nitriles, and nitroalkanes. To provide a comprehensive analysis, we compared the relative performance of AdQPhos-based precatalysts with other state-of-the-art catalysts for each class of reactions. Remarkably, AdQPhos-based precatalysts showed superior catalytic performances, surpassing the activity of other precatalysts in all classes of α-arylations. This accomplishment met our primary objective of developing a general class of ligand/precatalysts for these cross-coupling reactions. Additionally, we achieved our secondary goal, as these reactions demonstrated operational excellence both in traditional organic solvents and environmentally benign aqueous micellar conditions. The key findings in this regard are discussed in the following sections.
α-arylation of oxoindoles
The α-functionalization of cyclic amides, especially oxoindoles, is notably significant, primarily through the α-arylation method, which facilitates direct synthesis of C-3 arylated oxoindoles.62,63 These structural motifs are widely distributed in various natural products and pharmaceutical targets,62,63 including anti-HIV agents,62 and anti-tumor compounds.63 Despite this significance, such palladium-catalyzed C-3 arylations have not been extensively explored in comparison to other types of α-arylations.64–66
The first such exclusive study on oxoindoles was reported by Willis and co-workers in 2008.64 Later, Buchwald reported an improved approach with the development of the XPhos based catalytic system.65 Until now, only <10 reports are available on this topic despite the significant interest from medicinal chemists. 64–66 In addition, to the best of our knowledge, there are no existing literature reports on the usage of micellar technology for the palladium-catalyzed α-arylation of alkyl or cyclic amides. Taking this as a technological challenge, we decided to explore our AdQPhos based system for these reactions under milder aqueous conditions.
We used 4-bromo-N,N-dimethylaminobromobenzene (3a, 242950) as the model substrate with N-methyl-2-oxoindole (3b, 466921) as the nucleophile with NaOtBu (703788) as the base in conjunction with PS-750-M (911178) as the surfactant with water as the reaction medium (Figure 4). Our rationale behind utilizing PS-750-M micelles vs. other commercially available surfactants such as TPGS-750-M (763896), Brij-30, lies in their higher inner-core polarity,37 which is crucial for solubilizing polar amide enolates. For comparison studies, we performed the standard reaction using various AdQPhos Pd precatalysts, namely AdQPhos Pd G3 2a, AdQPhos Pd G4 2b, AdQPhos Pd G6 2c, and AdQPhos Pd(crotyl)Cl 2d, as well as several other state-of-the-art precatalysts (see Table 1 for the % yields). Gratefully, all the AdQPhos-based precatalysts (2a, 2b, 2c, and 2d) gave the desired product smoothly with excellent conversions. No double arylation side products were observed. The palladacycles 2a and 2b yielded 80% and 86% of the desired product (3c), respectively (entries 1 and 2). As expected, the oxidative addition complex 2c and crotyl variant 2d exhibited exceptional activity, yielding 99% conversion to the desired product with 92% isolated yield (entries 3 and 4). This could be attributed to the easier generation and consumption of active “L1Pd(0)”. In contrast, the QPhos Pd G3 (903027) or crotyl variants gave inferior conversions to the desired product (31% and 50%, respectively, entries 5 and 6). This clearly demonstrates the superior catalytic activity of the adamantyl (Ad) substituted P over the corresponding t-butyl analog. Other state-of-the-art precatalysts, such as XPhos Pd G3 (763381) gave moderate conversion (59%, entry 7), whereas (dtbpf)PdCl2 (701602) and DavePhos Pd G3 (804959), exhibited poor conversions (traces and <5%) under similar conditions (entries 8 and 9). Interestingly, t-BuXPhos Pd G3 (762229) demonstrated better activity with an 83% yield of the 3c under standard conditions (entry 10). Replacing the PS-750-M with another non-ionic surfactant (2 wt% TPGS-750-M aqueous solution) yielded inferior conversions to 3c (< 39%, entry 12).
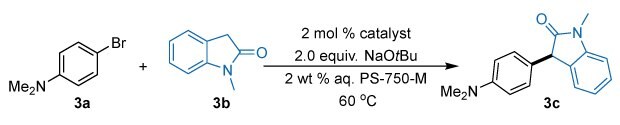
Figure 4.Synthesis of 3c (3-(4-(Dimethylamino)phenyl)-1-methylindolin-2-one) from 4-bromo-N,N-dimethylaminobromobenzene and N-methyl-2-oxoindole with NaOtBu in conjunction with PS-750-M.
Conditions: 3a (0.55 mmol), 3b (0.5 mmol), [Pd] (2 mol %), NaOtBu (1.0 mmol), 1.0 mL 3 wt % aq. PS-750-M, 60 °C, 20 h (all conversions are based on 1H NMR.
The superiority of PS-750-M could be due to the higher polarity of its micellar inner core. From a sustainability perspective, we performed the same reaction under organic solvent conditions (1,4-dioxane). Only 58% conversion was observed with 2d as the catalyst (entry 11), further highlighting the sustainability factor of our AdQPhos-based catalysts in α-arylation of amides.
Further, the scope of this technology was subsequently explored (Figure 5, 3c-3r). While expanding the scope, we ensured to test the activity of the catalysts 2d under both sustainable (aqueous micellar) as well as the non-sustainable (1,4-dioxane) conditions. Under micellar conditions, the reactions with 5-bromoindole (B68607) containing free N-H gave 67% of the desired C-3 arylated product without any side reactions (3d). Whereas under organic solvent conditions, the yield was almost reduced to half (33%). This was primarily due to the unwanted side reaction at the “N” center. The sterically demanding 2-bromomesitylene (B71608) also reacted smoothly with nucleophile 3b, yielding 80% of the desired product 3e. Similar to the previous example, the reaction under non-sustainable conditions only gave 46% of the desired product. Interestingly, when 3b was reacted with the more sterically challenging substrate bromo-tri-isopropylbenzene (637939) the reaction yield remained excellent (92%, 3g). Furthermore, electron-rich aryl halides such as N,N-dimethylbromobenzene, 4-bromomorpholinyl benzene, 4-bromoanisole, 4-bromo tert-butyl benzene were successfully introduced to the C3 position of the oxoindole (3b) under these conditions with moderate-to-excellent yields (3c-92%; 3f-81%, 3i-86%, 3j-90%). While evaluating the solvent effect on this reaction, it became obvious that micellar technology outperformed the traditional dioxane-based reactions, highlighting the effectiveness of our catalyst system under sustainable aqueous micellar conditions. Notably, this catalyst was inefficient in enabling the desired cross-couplings of challenging quinoline substrates (3k, 3l), presumably due to the known catalyst deactivation by quinoline binding. Similarly, naked oxoindoles are not compatible with the current conditions due to the formation of obvious side products in considerable quantities (3m, 3n).
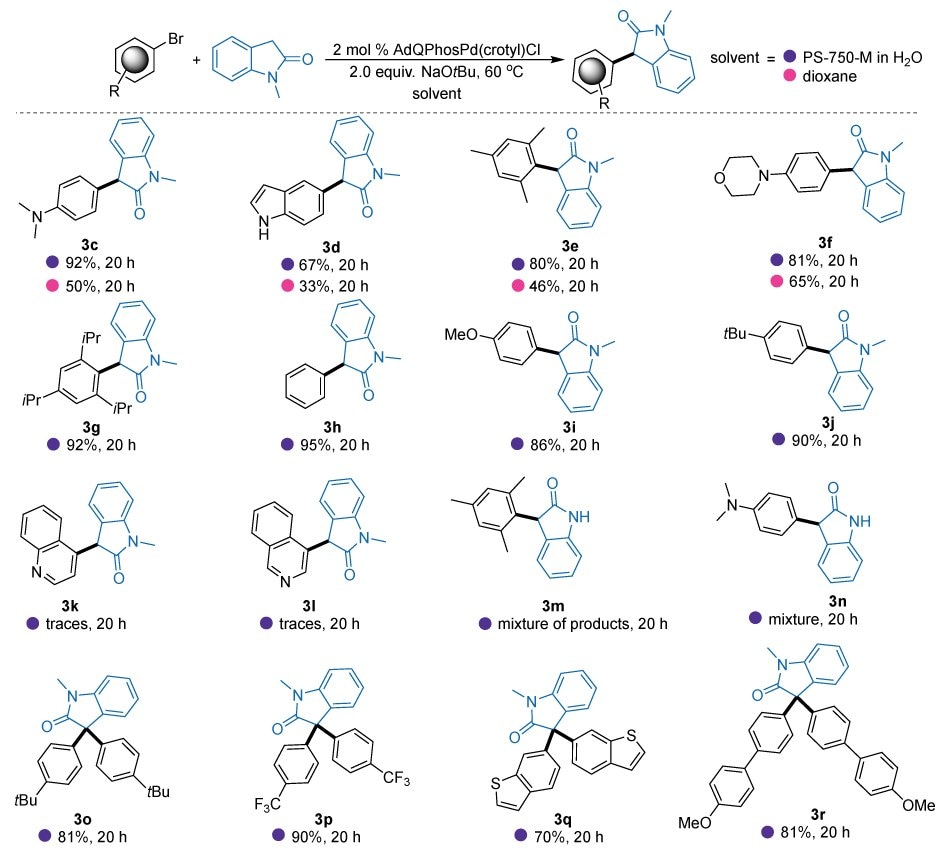
Figure 5.α-arylation of N-methyl-2-oxoindole (3b)a with different aryl halides using the catalyst AdQPhos Pd(crotyl)Cl (2d) in reaction mediums of PS-750-M in water and 1,4-dioxane.
aConditions: Ar-Br (0.55 mmol), Oxoindole (0.5 mmol), [Pd] (2 mol %), NaOtBu (1.0 mmol), 1.0 mL 3 wt % aq. PS-750-M, 60 °C, 20 h, isolated yield.
Moreover, we explored the scope of generation of quaternary centers through this strategy with double arylation of 3b. Compared with the α-arylation of 2° enolates as discussed above, the reactions utilizing 3° enolates as nucleophiles to synthesize 4° carbon centers through the palladium-catalyzed α-arylation reactions are considerably more challenging due to steric factors associated with the enolates, which limit their transmetalation to the palladium center.9 With slightly increased catalyst and base loading (catalyst, 3 mol%; base, 3 equiv.), the α-arylation reactions went smoothly with decent isolated yields of the desired diarylation products (Figure 5, 3o-3r). For instance, irrespective of the electronic nature of the electrophiles, products 3o, 3p, and 3r were isolated with excellent yields (81%, 90%, and 81%, respectively). This approach also accommodated heterocycles like benzothiophene with moderate yield (3r, 70%).
Other α-arylations
Following the successful implementation of micellar α-arylations for oxoindoles, our research sought to explore the applicability of AdQPhos-based precatalysts in other α-arylations. In this direction, we employed enolates derived from phenyl acetonitriles, nitroalkanes, esters, and ketones. Our objective was to evaluate the catalytic efficacy of these precatalysts in comparison with the established catalysts. Through these extensive comparative studies, we aimed to ascertain the performance enhancements offered by our precatalysts, thereby underscoring their potential significance in advancing the traditional α-arylation methodologies.
We began our studies by studying the compatibility of phenyl acetonitrile-derived enolates for the Pd-catalyzed α-arylation reactions under aqueous conditions (Figure 6). In this context, pioneering reports from Hartwig's group had set foundations for designing new catalysts and reaction conditions.24 Following these lines, notable works from the Verkade67 and Crudden25 groups improved procedures to accommodate broader substrate scope under milder conditions. Recently, Gessner’s YPhos has shown similar reactivity for the Hiyama-Denmark coupling.68 However, most of these protocols encountered drawbacks:
- High catalyst loading due to catalyst poisoning due to the Pd-CN binding,
- Elevated reaction temperature (to promote reductive elimination),
- Need of stronger bases like LiHMDS (324620) and NaHMDS (235083), and
- Use of hazardous organic solvents as the reaction medium.
In addressing these challenges, recently, Handa and co-workers reported a micellar nanocatalytic approach based on PS-750-M,47 although the challenges of catalyst recyclability and synthetic reproducibility using nanocatalysis still persist. It is important to note that, based on our literature search, until today, there is not a homogenous catalyst system that could address all of the four challenges mentioned earlier. We were curious to see if our AdQPhos-based system could address some of these challenges.
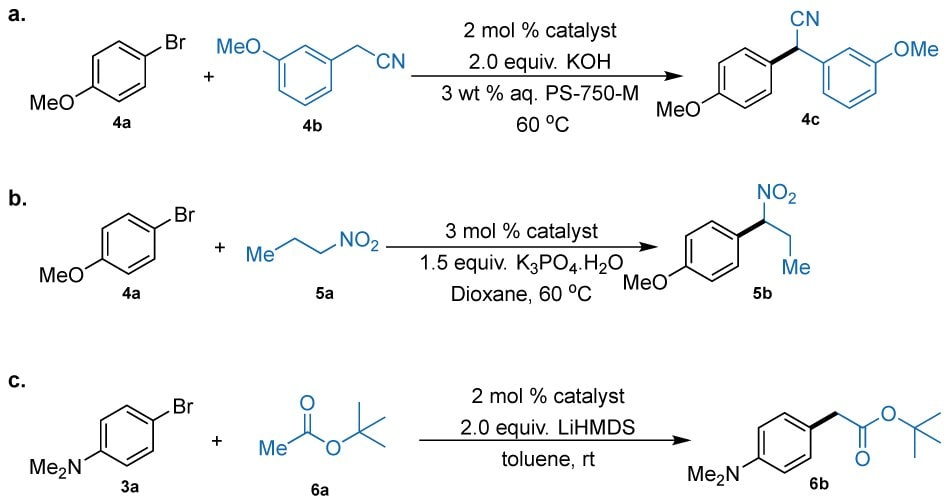
Figure 6.(a) α-arylation of 3-methoxy phenyl acetonitrile 209392 (4b); (b) α-arylation nitropropane (5a); (c) α-arylation of tert-butyl acetate (6a).
We started our optimization studies employing 3-methoxy phenyl acetonitrile (209392, 4b) as the nucleophile, 4-bromoanisole (B56501, 4a) as the electrophile, KOH as the base, and aqueous micelles of PS-750-M as the reaction medium (Figure 6, Part (a)). To showcase the effectiveness of our AdQPhos-based system over others, we also performed an extensive control study with other catalysts. To our delight, the desired product, 2-(3-methoxyphenyl)-2-(4-methoxyphenyl)acetonitrile 4c, was obtained in excellent yields with palladacycles 2a and 2b (89% and 94%, respectively; Table 2, entries 1 and 2). The reaction was further improved to 98-99% with the crotyl or G6 variants (entries 3 and 4). Inconsistent with the previous optimization studies, the reaction yields dropped substantially while using QPhos palladacycle or its crotyl variants (60% and 82%, respectively; entries 5 and 6). The conversion to the desired product 4c dropped to 59% when the bisphosphine-based (dtbpf)PdCl₂ was used as the catalyst (entry 7). The reaction yields remarkably improved when XPhos Pd G3 was used (88%, entry 8). This comparative study indicated the superiority of AdQPhos-based precatalysts over traditional biaryl or other types of state-of-the-art phosphines.
Further, the suitability of our AdQPhos systems in the α-arylations of nitroalkane was studied and compared to the traditionally employed catalytic systems (Figure 6, Part (b)).23,69 This particular type of α-arylation has been relatively underexplored, although the resulting products are the key precursors for accessing functionalized secondary/tertiary amines. Previously, the Buchwald and69 Kozlowski23 groups independently reported conditions for this transformation with some critical insights on mechanistic pathways. However, these protocols suffered serious limitations such as (a) higher [Pd] loadings (up to 10 mol%), (b) toxic organic solvent usage, and (c) operational difficulty where the experiments were performed under the glove-box conditions. Later, in 2017, Handa and co-workers reported an improved technology by introducing micellar catalysis.49 However, their method only explored the nitroethane and nitropropanes as the nucleophiles, and the reaction yields with nitropropane were moderate for most substrates studied therein.
In our current study, we started our studies using 4-bromoanisole (4a) and nitropropane (8.06851, 5a) as the model coupling partners with K3PO4 as the base under organic solvent conditions (1,4-dioxane) with lowered catalyst loading (3 mol %) in comparison to those reported (up to 10 mol %). The catalytic activity of the AdQPhos Pd-precatalysts was compared with other state-of-the-art precatalysts. Notably, the desired reaction proceeded smoothly without double arylations when employing AdQPhos precatalysts (2a, 2b, 2c, and 2d) (Table 3). Although under the standard reaction conditions with palladacycles 2a and 2b moderate yields (57% and 59%, respectively) of the desired product 2-(4-methoxyphenyl)butanenitrile 5b (entries 1 and 2) were observed, the G6 or crotyl variants of AdQPhos exhibited excellent activity with an impressive 99% conversion to the desired product (96% isolated yield, entries 3 and 4). Similar to the previous α-arylation with amides, the QPhos-based Pd G3 or crotyl variants only gave <10% of 5b, showcasing the remarkable superiority of our AdQPhos-based catalyst system (entries 5 and 6). Buchwald's XPhos Pd G3 gave moderate conversion (75%), whereas DavePhos Pd G3 gave only traces of 5b (entries 7 and 8). The bidentate ferrocene-based precatalyst, (dtbpf)PdCl₂, also gave poor conversions (28%) under similar conditions (entry 9). Interestingly, t-BuXPhos Pd G3 showed excellent reactivity, resulting in a 97% yield of the desired product 5b under these conditions (entry 10). With the effort to replace the hazardous solvent, 1,4-dioxane in this case, with water using micellar catalysis, we conducted extensive screening studies to identify suitable micellar media. With the micelles of PS-750-M, utilizing 2d as the catalyst, we achieved a remarkably high yield (98%) of 5b under standard conditions (entry 11). In contrast, the use of t-BuXPhos Pd G3 under micellar conditions was ineffective, as the reaction yield dropped to <31% (entry 12) in comparison to the reaction in organic solvent (97%, entry 10). These extensive screening studies demonstrated the superior adaptability and activity of AdQPhos-derived precatalysts over other state-of-the-art catalysts for this class of reaction.
In order to broaden the scope of nucleophiles in the α-arylations enabled by AdQPhos and its pre-catalysts, we explored esters in this study (Figure 6, Part (c)). Such α-arylations reactions are one of the most challenging classes of reactions due to:
- Instability of enolate (decomposition to ketene or possible decarboxylation)70,71
- Ester hydrolysis under the reaction conditions19
- Catalyst poisoning due to the possible difficulties in the reductive elimination18
To overcome these challenges, current strategies often employ masked ester nucleophiles such as Reformatsky reagents,18 or, silyl ketene acetals, etc.72,13 These approaches, however, suffered from several limitations, including step inefficiency, reliance on hazardous reagents, and operationally challenging protocols.13,72 Consequently, a catalyst-enabled, economically feasible protocol for α-arylation reactions involving esters remains elusive despite the extensive presence of α-arylated esters in APIs,73,74 and natural products.4,75 In this direction, we started our studies by employing the precatalysts of AdQPhos for the α-arylation of a nucleophile, tert-butyl acetate (8.02189, 6a) with 4-bromo-N,N-dimethylaminobromobenzene (3a) using toluene as the reaction medium. To evaluate the performance of AdQPhos precatalysts, we compared them with other state-of-the-art catalysts (Table 4). The AdQPhos-derived precatalysts (2a, 2b, 2c, and 2d) gave 39%, 54%, 99%, and 80%, respectively, of the desired product tert-butyl 2-(4-(dimethylamino)phenyl)acetate 6b, without any detectable amounts of possible diarylated product (entries 1-4). It is evident that the Pd G6 version of the AdQPhos ligands showed excellent activity. In contrast, corresponding precatalysts derived from QPhos only gave trace yields (8% and 32 %, entries 5, 6) for 6b. Such a lowered conversion is somewhat anticipated: typically, to enable such reactions using QPhos-based catalysts, the Reformatsky-type reagents are used as the nucleophile. Other ferrocene-based bidentate ligands like dtbpf did not yield any detectable product (entry 7), reiterating the demand for a monodentate phosphine containing an Ad moiety on the phosphorus. We also benchmarked the reaction with other oxidative addition complexes of monophosphines, such as Buchwald's RuPhos Pd G6, which gave only 74% of the desired product (entry 8).
These extensive comparative studies demonstrated the versatility of AdQPhos-derived precatalysts in enabling α-arylations of a diverse array of nucleophiles as well as their superiority over traditionally employed state-of-the-art Pd-precatalysts. This key observation positions the AdQPhos systems as potentially universal catalysts for Pd-catalyzed α-arylation reactions. Following these studies, the scope of these desired transformations was evaluated under our optimized conditions. We started our substrate scope evaluations with the α-arylations of phenyl acetonitriles (Figure 7, Part (a), 4d-4h). During these studies, we performed the reactions under both aqueous and organic solvent conditions to gain insights into the solvent's influence on each α-arylation reaction. All the reactions proceeded with moderate-to-excellent yields, no dehalogenations or other side reactions were observed with the nitriles containing chloro groups (4d, 82%; 4h, 86%). Heterocycles such as 4-morpholinyl-containing aryl bromides readily reacted with nitriles to yield the desired products in good yields (4d, 82%; 4g, 81%). Electron-rich (4d, 4g) or deficient (4e, 4h) or neutral substrates (4f) participated in the desired reactions without causing any significant drop in yield. It is important to note that these reactions proceeded effectively only under aqueous conditions. Under the conventional organic solvent conditions (e.g. 1,4-dioxane), considerably lower amounts of products were formed (4d - 4h). This observation emphasizes the superiority of our AdQPhos in enabling sustainable Pd-catalyzed cross-coupling reactions by leveraging micellar catalysis.33
Further, we extended our substrate scope evaluations for the nitro alkane α-arylations. Similar to the nitrile α-arylation, we performed reactions under both aqueous and organic solvent conditions. Conspicuously, the reaction yields were superior under organic solvent compared to surfactant conditions for most substrates, although moderate to good yields were typically obtained from the reactions employing micellar technology. (Figure 7, Part (b), 5b-5f). Nevertheless, our AdQPhos-based catalysts exhibited remarkable versatility, accommodating a wide range of aryl bromides. The electron-withdrawing (5f), and electron-donating (5b-5e) functional group-containing substrates participated in the reaction effectively to yield the desired product in moderate to excellent yield. The reaction exhibited favorable outcomes when dealing with substrates bearing heterocycles (5e).
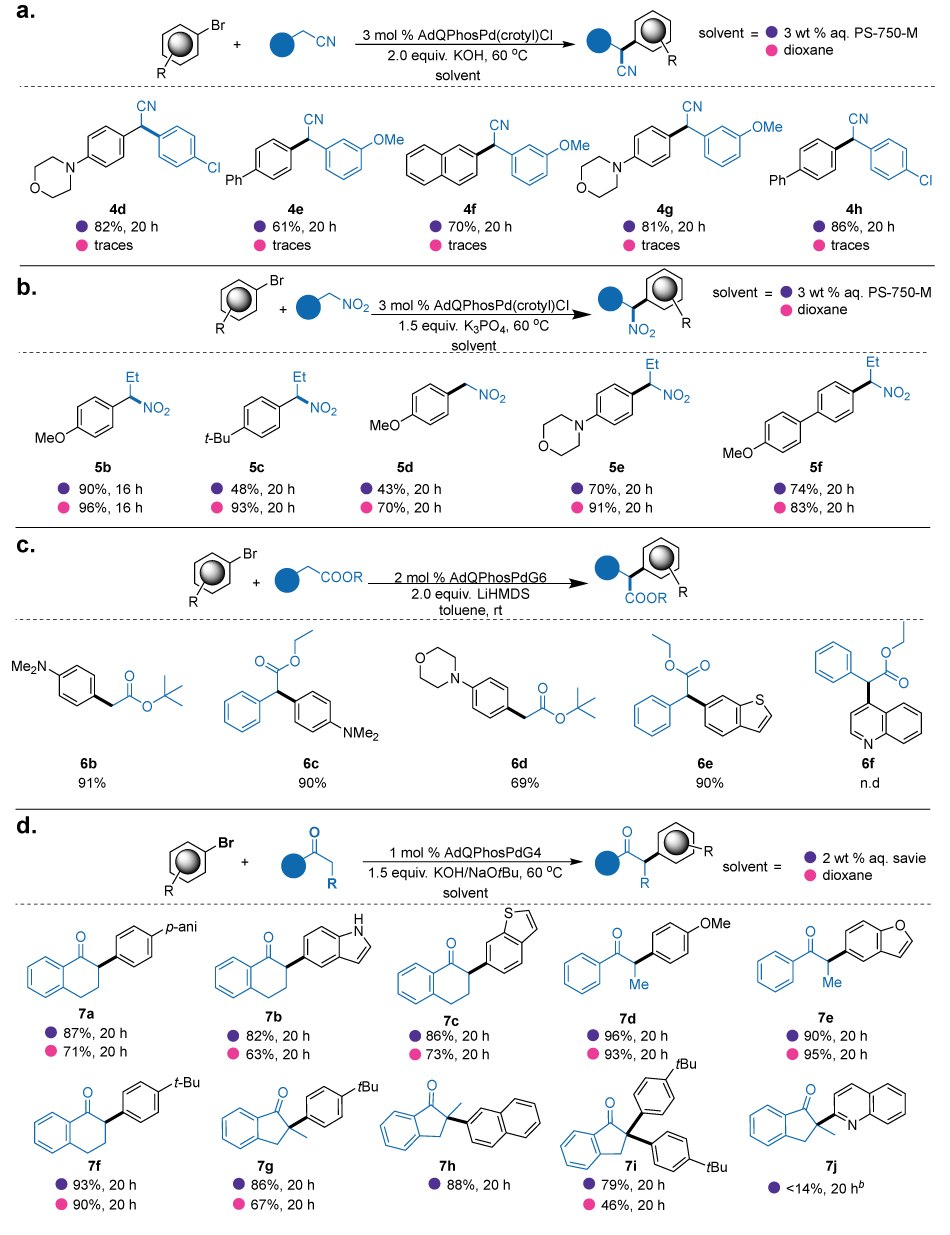
Figure 7.(a) α-arylation of nitriles, conditions: Ar-Br (0.6 mmol, 1.2 equiv.), ArCH2CN (0.5 mmol, 1.0 equiv.), [Pd] (3 mol%), KOH (1.0 mmol, 2.0 equiv.), 1.0 mL 3 wt% aq. PS-750-M or 1,4-dioxane 60 °C, 20 h (isolated yield) (b) α-arylation of nitroalkanes, conditions: Ar-Br (0.5 mmol), RCH2NO2 (2.5 mmol), [Pd] (3 mol%), K3PO4 (0.75 mmol), 1.0 mL 3 wt% aq. PS-750-M or 1,4-dioxane 60 °C, 20 h, (isolated yield) (c) α-arylation of esters, conditions: Ar-Br (0.5 mmol, 1.0 equiv.), RCH2COOR (1.0 mmol, 2.0 equiv.), [Pd] (2 mol%), LiHMDS (1.0 mmol, 2.0 equiv.), 2.0 mL toluene, 20 h (isolated yield) (d) α-arylation of ketones, conditions: Ar-Br (0.5 mmol or 1.1 mmole), RCH2COR (0.5 mmol), [Pd] (1 mol%), KOH or NaOtBu (0.75 mmol or 1.1 mmol), 1.0 mL 3 wt% aq. PS-750-M or 1,4-dioxane 60 °C, 20 h (isolated yield).
Next, the scope of α-arylation of esters was briefly evaluated by exploring different combinations of aryl halides and esters (Figure 7, Part (c), 6b-6e). It is important to note that under our reaction conditions, even with excess electrophiles, the reaction exclusively produced monoarylated ester products. Furthermore, these reaction conditions proved to be compatible with ethyl esters (6c, 6e), which are prone to base-mediated hydrolysis under typical reaction conditions. The reaction yields achieved with these conditions ranged from moderate to excellent, highlighting the versatility and effectiveness of our protocol, which accommodated electron-rich and heterocyclic aryl halides as electrophiles effectively. On the nucleophile side, the protocol demonstrated compatibility with both alkyl and benzyl esters. Notably, quinoline-based aryl halides seem to have issues under current conditions.
Additionally, the catalytic activity of AdQPhos or its precatalysts was evaluated for the α-arylation of ketones (Figure 7, Part (d)). Despite the numerous reports on the utilization of organic solvents, primarily employing hazardous solvents (1,4-dioxane or NMP),76 only a few studies have focused on enabling these reactions under milder and environmentally benign conditions, i.e., using water as the solvent.50 In this regard, recently, Lipshutz and coworkers developed a new technology under aqueous conditions by using [PdP(t-Bu)3Br]2 as a catalyst (up to 1 mol%), aqueous micelles of TPGS-750-M as a reaction medium, and KOt-Bu as the base.50 It is worthwhile to highlight that the Pd(I) dimer of t-Bu3P is one of the most unstable Pd catalysts; hence, it often causes issues in reproducing the results. With our bench-stable AdQPhos Pd G4 (up to 1 mol %), the reactions were carried out in water using a readily available base, KOH in conjunction with a new generation biodegradable surfactant, "Savie" developed by Lipshutz.77 A short substrate scope evaluation was performed to showcase the generality of this versatile catalyst (Figure 7, Part (d)). The reaction proceeded smoothly for cyclic (7a-7c, 7g-7j) and acyclic ketones (7d, 7e) with moderate-to-excellent yields. We continued assessing the solvent effect on catalysis by performing each reaction under aqueous and traditional organic solvent conditions. The coupling between cyclic ketone α-tetralone and sterically challenging 2-bromomesitylene proceeded smoothly to yield the desired product 7a with excellent yield under aqueous conditions (87%). Under organic solvent conditions, the reaction yield was only 71%, with the remaining unreacted starting materials (ketone and aryl halide). Furthermore, this approach was compatible with various heterocycles, including indoles bearing free N-H (7b), benzothiophenes (7c), and benzofurans (7e), with excellent isolated yields. Notably, unlike the α-arylation of nitroalkanes, the reactions on ketones proceeded more efficiently under micellar conditions than under traditional organic solvent conditions for most of the substrates, which, again, demonstrates the sustainability contributions from our AdQPhos-based system in the α-arylation chemistry.
Combining all these studies, we demonstrated that the AdQPhos-based system is a general, practical catalytic system for the palladium-catalyzed α-arylation reactions under organic solvent as well as under green aqueous conditions.
Scalability and Sustainability Score
To demonstrate the scalability of our AdQPhos-based technology for potential commercial applications, we picked the amide α-arylation reactions and performed them at a pentagram scale.
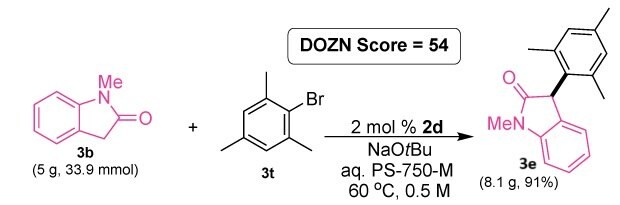
Figure 8.Gram-scale example. Synthesis of 3-mesityl-1-methylindolin-2-one 3e from N-methyl-2-oxoindole 466921 and 2-bromomesitylene B71608.
Conditions: 3b (33.9 mmol), 2-bromomesitlyene (35.7 mmol), AdQPhos Pd(crotyl)Cl (0.678 mmol), NaOtBu (67.8 mmol), 68 mL 3 wt% aq. PS-750-M, 60 °C, 20 h.
The expected α-aylated amide product, 3-mesityl-1-methylindolin-2-one 3e, was isolated in 91% yield, highlighting the usability of our AdQPhos-based system in reaction scale-up. The sustainability score of this reaction was evaluated using DOZN™.78 As the reaction was conducted under micellar conditions, the environmental impact of this transformation was remarkably lower compared to the traditional methods, and this was clearly reflected in the lower DOZN™ score of 54.
Conclusion
In summary, we have developed a new class of polyarylated diadamentylphosphino ferrocene (AdQphos) and its precatalysts as versatile catalytic systems for the Pd-catalyzed α-arylation reactions. The ligand synthesis is simple, scalable, and does not involve tedious workups or purification techniques. The scope and superiority of this catalytic system were demonstrated for a wide variety of nucleophiles, including nitroalkanes, nitriles, amides, and ketones. Most importantly, by incorporating catalysis in water under micellar conditions, the AdQphos-based catalytic system demonstrated the feasibility of enabling α-arylation reactions under milder aqueous conditions by replacing toxic/hazardous solvents such as 1,4-dioxane or NMP with some exceptions, bringing modern organic synthesis closer to being ideally sustainable.
References
To continue reading please sign in or create an account.
Don't Have An Account?