过程分析技术(PAT)通过实时监测和控制过程,确保生物制药生产的质量。它利用分析工具来开发制造工艺,以适应材料和设备的可变性。一旦确定了影响关键质量属性 (CQAs) 的关键工艺参数 (CPP),就会采用分析方法来监测和控制 CPP,使其保持在所需的设计空间内。这种方法将质量源于设计 (QbD) 原则融入工艺流程中,而不是最终才依赖产品测试。
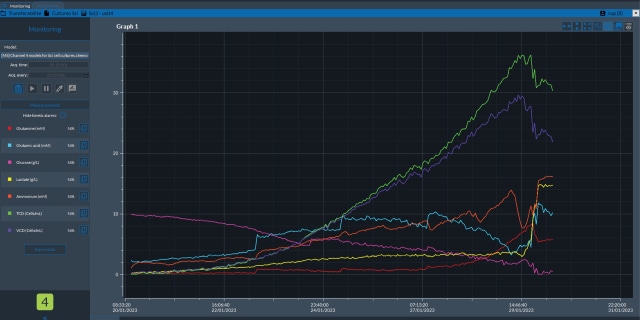
在线和实时拉曼监测
与传统的离线分析方法(如细胞培养多重测试分析仪、细胞活力分析仪或 HPLC)相比,拉曼光谱为上游 (USP) 和下游 (DSP) 过程提供了明显的优势,包括
- 使用单个探针进行多属性监测
- 细胞培养过程中自动喂料策略的实施
- 在单个光谱中收集完整的化学和生物信息
- 获得实时和原位测量
- Non-destructive analysis
- Easy implementation using an in-line probe or on-line, at-line, off-line mode as needed

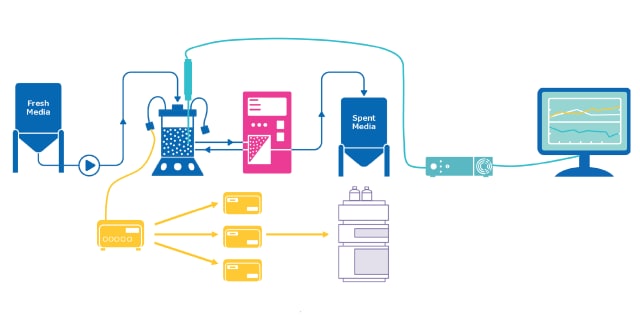
ProCellics™拉曼分析仪专为生物工艺行业设计,是一种易于使用的工艺分析技术 (PAT) 平台,用于监控从工艺开发到生产的 USP 和 DSP 生物工艺,进行定性和定量分析。此外,与手动取样相比,该平台还有助于节省时间、提高操作灵活性并降低污染风险和批次失败率。

ProCellics™拉曼分析仪有两种配置,完全适应生产要求,可满足从工艺开发到生产的一系列监测需求:
单通道装置只需一个探针即可监测培养物。套件包括一个带 785 nm 激光器的基本单元、一个探针、附件和一台包括 Bio4C® PAT 拉曼软件的一体式计算机。

多通道装置可在同一系统内并行监测多达四个培养物。该套件包括一个带 785 nm 激光器的基本单元、一个带四个探针的多通道单元、附件和一台包括 Bio4C® PAT 拉曼软件的一体式计算机。
先进的探针管与特定的软件功能相结合,可抵消光干扰的影响,无需遮挡生物反应器的光线。
它加强了拉曼技术在各种单元操作中的可扩展性,允许从一次性使用系统无缝过渡到多次使用系统,以及从工艺开发到生产的不同规模。
相关类别
我们的现成和可定制的生物加工细胞培养基 (CCM) 产品可提高上游 mAb、疫苗、基因/细胞治疗工艺的生产率。
Mobius®生物反应器系列包括台架规模(2 mL 和 3 L)、中试规模、临床规模和实验室规模。
利用我们的细胞保持解决方案提高灌流种子培养系的效率。实时监测和控制。
相关资源
- Direct Monitoring of Upstream Bioprocesses Using Raman Ready-to-Use Embedded Models and Transfer Learning Algorithm
Learn how ready-to-use Raman models simplify calibration and allows real-time monitoring of CPPs and CQAs in upstream processes - no custom calibration required. See results from upstream and downstream runs and how transfer learning can fine-tune model accuracy after a single batch.
- Application Note: In-Line Monitoring of CPPs and Capsid Titer in Upstream AAV Process with ProCellics™ Raman Analyzer
Explore the ProCellics™ Raman Analyzer for in-line monitoring of critical process parameters (CPPs) and capsid titer during AAV production in Human Embryonic Kidney (HEK) cell bioreactors.
- Data Sheet: ProCellics™ Raman Analyzer with Bio4C® PAT Raman Software
Specifically designed for the bioprocessing industry, ProCellics™ Raman Analyzer with Bio4C® PAT Raman Software enables you to perform in-line and real-time measurement of CPPs and CQAs, from process development to manufacturing.
- Application Note: Enabling accelerated Raman model calibration for seamless and reproducible real-time monitoring by combining Raman and automated sampling technologies
Explore how automated sampling can accelerate the integration of a Raman analyzer for a bioreactor application, speeding up the Raman model building phase, and facilitating in-line, real-time monitoring of critical process parameters (CPPs) and critical quality attributes (CQAs) during the manufacturing process.
- Application Note: Monitoring bioprocesses in a light environment using Raman spectroscopy
This application note introduces an advanced solution to facilitate the implementation of Raman technology in laboratory and manufacturing scales, in a normal light environment, to ensure the consistent monitoring of critical process parameters (CPPs) and critical quality attributes (CQAs).
- Application Note: An innovative approach to streamline Raman implementation for cell culture processes: One-batch calibration
This novel modeling approach eliminates the need for multiple calibration batches during the Raman implementation, allowing for a more streamlined and efficient workflow.
- Application Note: Real-time and in-line Raman Spectroscopy: A Window into Glycosylation Quality Analysis
This case study demonstrates the effectiveness of Raman technology for monitoring monoclonal antibody (mAb) glycan profiles during fed-batch processes, and how robust predictive models facilitate monitoring of glycosylation profiles.
- Application Note: In-line Real-time Monitoring of CHO Cell Culture Process Parameters Using Raman Spectroscopy
Cell culture processes are complex and highly variable and yet only a handful of key parameters such as temperature, pH, and dissolved oxygen (DO) are typically controlled in real time.
- Application Note: Implementation of Raman Spectroscopy for In-line Monitoring of CPPs of CHO Cell Perfusion Cultures
This application note introduces a case study for the implementation of a Raman spectroscopy soft-sensor for in-line and real-time monitoring of critical process parameters (CPP) in mammalian perfusion cell cultures.
- Application Note: Seamless Integration of Glucose Control Using Raman Spectroscopy in CHO Cell Cultures
Process analytical technology (PAT) and quality by design (QbD) are used in the biopharmaceutical industry to ensure quality is designed into a process and to achieve innovative quality improvements.
- Application Note: Cell-free modelling approach for efficient cell culture monitoring using Raman spectroscopy
The novel synthetic model approach provides a significative gain of time and resources for the Raman calibration phase which is reduced to just a few days.
- Article: One-Batch Calibration: A Process-, Instrument-, Scale- & Site-agnostic Method For Streamlining Raman Implementation
Challenges associated with Raman spectroscopy implementation in bioprocesses can be overcome by the innovative one-batch calibration approach. This streamlined process requires less time and resources compared to traditional approaches, unlocking Raman benefits for biopharmaceutical applications.
BioContinuum™平台:未来生物制造设施的推动者!
如要继续阅读,请登录或创建帐户。
暂无帐户?