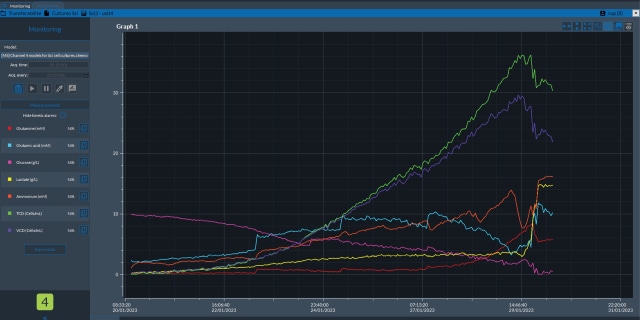
过程分析技术(PAT)通过实时监测和控制过程,确保生物制药生产的质量。它利用分析工具来开发制造工艺,以适应材料和设备的可变性。一旦确定了影响关键质量属性 (CQAs) 的关键工艺参数 (CPP),就会采用分析方法来监测和控制 CPP,使其保持在所需的设计空间内。这种方法将质量源于设计 (QbD) 原则融入工艺流程中,而不是最终才依赖产品测试。
自动无菌采样
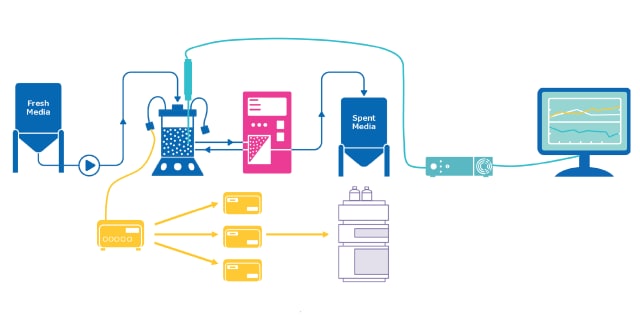
自动无菌采样尤其适用于上游工艺(USP)的开发,因为它在细胞培养样品的采集方面具有显著优势,但该技术也可用于下游工艺(DSP)和微生物发酵。使用这种 PAT 技术,无需人工干预即可从源头采集样品,并直接运送至分析或储存场所,同时保持封闭、无菌的采样环境。自动无菌采样技术不分模式,采用模块化设计,在实施过程中可提供灵活的定制解决方案。该技术还可与拉曼光谱等其他 PAT 技术相结合,以改进数据驱动模型的校准和验证。
与目前的离线采样和分析程序相比,自动无菌采样具有以下明显优势:
- 降低样品源的污染风险
- 增加采集具有过程代表性的数据点的频率和数量
- 延长实验与分析行动之间的周转时间
- Minimized number of invariabilities associated with off-sample hold times and testing
- Decreased number of experiments required to make key process decisions

MAST®自动采样解决方案是一种多功能、模式无关的无菌自动采样解决方案,可确保直接采集、递送和分析来自多个来源的样本,而无需人工干预,从而使用户有更多时间执行其他增值任务。
相关类别
我们的现成和可定制的生物加工细胞培养基 (CCM) 产品可提高上游 mAb、疫苗、基因/细胞治疗工艺的生产率。
Mobius®生物反应器系列包括台架规模(2 mL 和 3 L)、中试规模、临床规模和实验室规模。
利用 Cellicon® 细胞保持解决方案,使灌注过程实现更高的细胞密度。
相关视频
相关资源
- Datasheet: MAST® Autosampling Solution
The MAST® Autosampling Solution is a versatile & modality-agnostic aseptic autosampling solution for reliable on-line and near real-time sample analysis.
- Technical Article: Enhance Bioprocess Sampling with MAST® Autosampling Solution
The MAST® Autosampling Solution is a cutting-edge, modality-agnostic system that automates the collection and delivery of samples from multiple sources, significantly enhancing efficiency in biopharmaceutical processes. By minimizing manual intervention, it allows scientists to focus on value-added tasks while reducing contamination risks and accelerating analytical turnaround times.
- Technical Article: Improving Efficiency and Control in Biopharma Manufacturing With Process Analytical Technology
Process Analytical Technology (PAT) is transforming biopharmaceutical manufacturing by enabling real-time monitoring and control of processes, ensuring consistent product quality amidst variability. By integrating analytical tools and Quality by Design principles, PAT enhances efficiency and accelerates the development of innovative medicines while maintaining stringent quality standards.
- Technical Article: Accelerating Data Access in Bioprocesses with Automated Aseptic Sampling
Automated aseptic sampling is an innovative process analytical technology (PAT) that significantly enhances bioprocesses in monoclonal antibody manufacturing by reducing contamination risks and increasing measurement frequency. By streamlining sampling procedures, it allows for improved efficiency, better process understanding, and tighter control, ultimately supporting real-time bioprocess monitoring and quality assurance.
- Technical Article: Accelerating Process Development with Automated Aseptic Sampling
Automated aseptic sampling is a key advancement that accelerates process development in biopharmaceutical manufacturing by enabling frequent and reliable sample collection while minimizing contamination risks. This innovative approach enhances data accessibility and process understanding, leading to more efficient workflows and improved product quality throughout the development lifecycle.
- Technical Article: Facilitating Raman Model Calibration Using Automated Sampling Technologies
This page describes the use of an innovative workflow to expedite integration of in-line Raman measurement for a bioreactor application using automated sampling and data analysis. This approach accelerates the Raman model-building phase which then enables in-line, real-time monitoring of critical process parameters (CPPs) and critical quality attributes (CQAs) during bioprocessing.
- White Paper: Automated Aseptic Sampling for Accelerated Access to Process and Quality Data in Upstream Bioprocessing
This white paper focuses on PAT and the use of automated sampling technology to accelerate analytical and quality control methods and provide an approach for access to in-line data to monitor processes in real time.
- Application Note: Enabling Accelerated Raman Model Calibration for Seamless and Reproducible Real-Time Monitoring by Combining Raman and Automated Sampling Technologies
Explore how automated sampling can accelerate the integration of a Raman analyzer for a bioreactor application, speeding up the Raman model building phase, and facilitating in-line, real-time monitoring of critical process parameters (CPPs) and critical quality attributes (CQAs) during the manufacturing process.
- White Paper: Accelerate Process Development with Automated Aseptic Sampling
This whitepaper describes evaluation of the MAST® Autosampling Solution as part of an automated PAT system implemented by Takeda Pharmaceuticals.
BioContinuum™平台:未来生物制造设施的推动者!
如要继续阅读,请登录或创建帐户。
暂无帐户?