Recent Developments on Hydrogen Release from Ammonia Borane
Section Overview
- Introduction
- Background on Ammonia Borane
- Hydrogen Release Studies
- Solid State Thermal Decomposition
- Transition Metal Catalyzed Dehydrogenation of Ammonia Borane in Solution
- Dehydrogenation of Ammonia Borane in Ionic Liquids
- Solution Phase Thermal Dehydrogenation
- Nanophase Ammonia Borane in Mesoporous SBA-15
- Summary
- Acknowledgments
Introduction
Record crude oil prices combined with public interest in energy security have resulted in increased attention to a potential transportation economy based on hydrogen fuel. One of the greatest challenges is the discovery and development of materials and compounds capable of storing enough hydrogen on-board to enable a 300-mile range without adding significant weight or volume to today’s conventional automobile. A minimum of five kilograms of hydrogen would need to be stored on-board to drive even the most fuel-efficient vehicle three hundred miles. At standard temperature and pressure (STP), five kilograms of hydrogen requires a volume of nearly 54 m3. Unfortunately, even highly compressed hydrogen gas is unlikely to be of sufficient volumetric density (40 g/L at 700 bar) to enable a fuel system to meet the 300-mile target. Liquified hydrogen at 70 g/L also falls short of reasonable volumetric system targets when cooling systems required to keep hydrogen liquified (b.p. –253 °C) are added to the storage system.
Many materials with high volumetric and/or gravimetric hydrogen densities have been studied over the last few years, especially those composed of the lighter elements. The driving force for focus on light elements arises from the United States Department of Energy (US DOE) targets for on-board hydrogen storage as outlined in the article by Sunita Satyapal et al. in this issue. It is important to remember that the DOE targets, 60 gm H2/kg system and 45 gm H2/L system in 2010 include not just the material, but the entire storage system. The storage system is considered as all storage and hydrogen conditioning components leading up to the fuel cell.1
Our group and others have been interested in chemical hydrogen storage materials that use the elements nitrogen and boron to chemically bind hydrogen. In these chemical hydrogen storage materials, hydrogen is ‘discharged’ by a chemical reaction and the hydrogen is ‘recharged’ by a chemical processing pathway. This makes them unique compared to metal hydride materials or carbon sorbent materials where the hydrogen release and uptake is controlled by temperature and pressure. One compound in particular, ammonia borane (AB = NH3BH3) has received significant interest given its stability and commercial availability. Ammonia borane, isoelectronic with ethane, is a solid at room temperature, stable in air and water and contains 190 g/kg (100–140 g/L) hydrogen. Figure 1 shows that if a large portion of the hydrogen can be liberated, AB has a higher gravimetric density than most other reported chemical systems. This capacity coupled with stability has resulted in renewed interest in studying ammonia borane as a hydrogen storage material. While the material would not be regenerated “on-board” it could potentially meet many DOE targets.

Figure 1. Comparison of gravimetric and volumetric densities of various hydrogen storage materials.
Background on Ammonia Borane
The first published research describing the properties of these AB hydrogen rich materials was funded in large part by U.S. government agencies interested in boron-based jet fuels.2 Ammonia borane was first synthesized and characterized by Sheldon Shore in the 1950s during his thesis research in Richard Parry’s lab at the University of Michigan.3 They performed a series of clever experiments designed to identify the ‘mysterious’ B2N2H6 adduct, the so-called diammoniate of diborane (DADB), formed when ammonia was mixed with diborane. The same adduct appeared to be formed when an ammonium salt was mixed with a metal borohydride in liquid ammonia. Previous work had suggested that the reaction produced an ammonium cation and the corresponding diborane anion [NH2(BH3)2][NH4]. However, there was no unambiguous evidence to rule out the formation of the isomeric borohydride salt [BH2(NH3)2][BH4].3,4
One of the key experiments used to elucidate the true structure of DADB was to add an ammonium salt (NH4Cl) to DADB. If the structure of DADB was [NH4][BH3NH2BH3] then no change in reactants would be observed, however, if DADB were the alternate isomer consisting of a borohydride anion, [BH2(NH3)2][BH4], then an exchange reaction would occur to yield [BH2(NH3)2]Cl + NH4BH4. When Shore and Parry performed the reaction they did see the predicted chloride salt, but they also observed a new material that proved to be NH3BH3 (Equation. 1).3

Equation. 1
Subsequent work showed that NH4BH4 was unstable and decomposed to ammonia borane and hydrogen with a half life of hours at room temperature.5
Alternate pathways have been developed to simplify the synthesis and increase the yield of ammonia borane. NH3BH3 can be prepared by a reaction of alkali metal borohydride and ammonium chloride in diethyl ether at room temperature. (Equation. 2) Similar reactions are used to synthesize alkylaminoboranes such as (CH3)3NBH3. Additionally NH3BH3 can be prepared by a reaction of diethyl ether-borane with ammonia in yields of up to 70% (Equation. 3).

Equation. 2,3
A simple molecular description of NH3BH3 shows that it is a donor-acceptor adduct formed as a result of the dative bond between a Lewis acid (BH3) and a Lewis base (NH3). The compound is a solid at room temperature primarily due to di-hydrogen bonding and dipole-dipole interactions.6 Though ammonia borane and diammoniate of diborane have the same chemical formula they are very different in stability. Ammonia borane is soluble in ether while the diammoniate of diborane is not. Ammonia borane is not readily hydrolyzed by water while diammoniate of diborane reacts instantaneously with water. Over time solid DADB slowly converts to solid AB at room temperature. Thus AB is more readily applicable than DADB to hydrogen storage for automotive use.
Hydrogen Release Studies
Recent work has shown that NH3BH3 can release more than 2 moles of H2 with heating to modest temperature. The reactions of hydrogen evolution can be summarized as shown in Equation. 4 through 7.
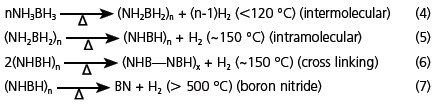
Equations. 4,5,6,7
The first two steps, reaction of AB to form PAB, polyaminoborane, (NH2BH2)n; and PAB to form PIB, polyiminoborane (NHBH)n amount to 12 mass% hydrogen. The hydrogen release in these first two steps occurs at temperatures less than 150 °C. At a slightly higher temperature the cross-linking between molecules is observed to release additional hydrogen. These materials are common intermediates used as precursors to boron nitride, which is formed at temperatures much greater than 500 °C.7–10
Efficient methods are needed to dehydrogenate ammonia borane to release hydrogen at moderate temperatures and reasonable rates. Various groups have used different methods to study and catalyze the dehydrogenation reaction. Here we review five different approaches used to release hydrogen from ammonia borane.
- Solid state thermal decomposition
- Transition metal catalyzed dehydrogenation
- Ionic liquid catalyzed dehydrogenation
- Solution phase thermal decomposition
- Nanophase ammonia borane encapsulated in SBA-15
Solid State Thermal Decomposition
Geanangel was one of the first researchers to report studies on the thermal dissociation of AB using thermomanometry, pyrolysis, DSC, DTA and TGA in the early to late 1980s.11–13 The DTA curves showed a sharp endothermic peak beginning at just above 112 °C, which corresponds to the melting point of pure AB (112–114 °C). On further heating thermomanometry showed a sharp pressure rise near 120 °C due to hydrogen evolution. That corresponded to the first exothermic peak in the DTA at 117 °C, which accompanies vigorous decomposition as can be seen by the mass loss in the TGA data. Upon further heating the rate of pressure increase slowed as the hydrogen evolution from the first step reached completion. Continued temperature increase resulted in the release of a second equivalent of H2.
In more recent studies, Wolf and co-workers in Freiberg, Germany have revisited the thermal analysis of AB using controlled calorimetric studies to identify the events leading to dehydrogenation and isolation of products obtained in the process. They also explored the effect of experimental conditions such as heating rate and pressure on the thermal decomposition of AB.7–9 As expected even high overpressure of hydrogen neither significantly impacted the hydrogen release rate nor the enthalpic parameters during the thermal decomposition since both the transitions (AB --> PAB and PAB --> PIB) are exothermic. Furthermore, there was little change in the quantities of volatile products generated. On the other hand, they report that the rate of heating appears to have a measurable impact on the thermal events and the volatile product formation. Higher heating rates resulted in greater quantities of volatile products such as borazine.7–9 The formation of volatile products in the absence of subsequent hydrogen purification will be problematic for PEM fuel cells that are easily fouled by contaminants in the hydrogen stream.
Transition Metal Catalyzed Dehydrogenation of Ammonia Borane in Solution
Chandra et al. reported the transition metal catalyzed dehydrogenation of AB under aqueous conditions and suggest that the activation process takes place on the metal catalyst surface.14,15 As a plausible mechanism, it is reasonable to consider that there should be interactions between the NH3BH3 molecule and the metal particle surface to form an activated complex which upon attack by a H2O molecule readily leads to a concerted dissociation of the B–N bond and hydrolysis of the resulting BH3 byproduct to produce the boric acid and H2. Interestingly, in the absence of H2O, dehydrocoupling between NH3BH3 molecules to form new B–N bonds occurs, probably via a closely related intermediate, on the metal surface. In their studies Pt supported on carbon was the most efficient catalyst for dehydrogenation. Alternatively Denney and Goldberg16 reported use of molecular complexes of transition metals (e.g. Ir) containing pincer ligands for dehydrogenation at room temperature in just 14 minutes, considerably faster than the previously reported rhodium complexes (dehydrogenation of dimethyl amino borane required 2–4 days at 45 °C).17 The plausible reason for this difference in rates is homogeneous vs. heterogeneous catalysis wherein the Ir complex catalyzes the dehydrogenation by a homogeneous pathway while the rhodium catalyst undergoes a transformation to a rhodium colloid or cluster. It is interesting to note that most of the product obtained as a result of dehydrogenation is a pentamer having the formula (BH2NH2)5 (Equation. 8).

Equation. 8
Here the catalysis is performed by (POCOP)Ir(H)2; (POCOP) is [h3-1,3-(OPtBu2)2C6H3]).
Linehan17 reported that a Rh catalyst has been shown to release one third of the available H2 from dimethylamino borane ((CH3)2NHBH3) under ambient conditions. It is thus important to not only determine the active form of the catalyst for this reaction but to also understand, in detail, the reaction pathway and kinetics for the amine borane dehydrocoupling. Earlier studies proposed that Rh nanoparticles were the catalytic species as determined from ex situ measurements of the Rh species. X-ray absorption fine structure (XAFS) and NMR demonstrated that nanoparticles are not present during this reaction but rather, small Rhn clusters (n=4–6) are the predominant species during dehydrocoupling. Such in situ techniques probe the catalyst not only in the reaction solvent and at the reaction pressure and temperature, but also while it is undergoing chemical reactions with the reactants in the system. In situ XAFS and NMR are especially useful for following the reaction pathways and kinetics of the reactants, the products and for catalyst characterization (Figure 2).
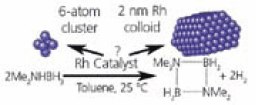
Figure 2. Schematic pathway of formation of Rh clusters during the dehydrogenation of dimethylamino-borane (DMAB). 17
Dehydrogenation of Ammonia Borane in Ionic Liquids
Ionic liquids are salts that are liquid at temperatures below 100 °C. These salts have unique properties that make them attractive substitutes for organic solvents in hydrogen storage systems, including: (1) negligible vapor pressures; (2) stable to elevated temperatures; (3) able to dissolve a wide range of compounds and gases; (4) weakly coordinating anions and cations that provide an inert reaction medium which can stabilize polar transition states; and (5) recyclable with little loss of activity.
Bluhm and Sneddon recently reported dehydrogenation of AB in ionic liquids such as 1-butyl-3-methylimidazolium chloride [bmimCl] and compared it to solid-state dehydrogenation.18 In contrast to the solid-state reaction, ammonia borane dehydrogenation in bmimCl showed no induction period with hydrogen evolution beginning immediately upon placing the sample in the heated oil bath. Separate samples heated for only 1 h at 85, 90, and 95 °C evolved 0.5, 0.8, and 1.1 equiv of H2, while samples heated at these temperatures for 3 h produced 0.95, 1.2, and 1.5 equiv. Heating for 22 h gave a total of 1.2, 1.4, and 1.6 equiv of H2, respectively, which are values significantly greater than the 0.9 equiv ultimately obtained in the solid-state reactions. Including the bmimCl weight, the final values correspond to the evolution of 3.9, 4.5, and 5.4 wt % H2. The role of bmimCl in enhancing the rate and extent of ammonia borane dehydrogenation has yet to be proven, but it is significant that [(NH3)2BH2 +]BH4 – has also been reported to form polyaminoborane upon heating. (Figure 3) Ionic liquids are known to favor the formation of polar intermediates and transition states, and the observation that [(NH3)2BH2 +]BH4 – and/or BH4 – are produced in the bmimCl reaction within the first hour suggests that the activating effect of the ionic liquid may be related to its ability to induce formation of such ionic species.
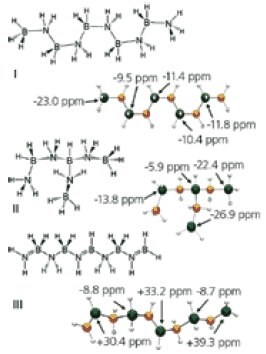
Figure 3. The possible structures arising from dehydropolymerization of AB using DFT/GIAO calculated values of 11B NMR.18
Solution Phase Thermal Dehydrogenation
The thermal decomposition of ammonia-borane in selected solvents was studied by Geanangel using 11 B NMR at temperatures up to and including reflux in various solvents. Solvents chosen dissolved NH3BH3 to a reasonable concentration and possess boiling temperatures of 80 °C, the lowest temperature at which ammonia-borane decomposes at an acceptable rate.19 Solvents such as alcohols, which undergo solvolytic reactions with ammonia-borane, were not included in the study. The purpose of the work was to identify solvents and conditions best suited for preparing and carrying out reactions of NH2BH2 generated thermally from ammonia borane. It was hoped that NH2BH2, which is subject to facile association in the solid phase, would be stabilized sufficiently by solvation to be at least detectable in solution. If not, the quantities of its oligomers detected in the reaction mixtures would indicate the extent of its formation. Three types of reactions were observed: (1) hydrogen-loss decomposition of NH3BH3 leading to cycloborazane(s) and eventually to borazine, (2) base displacement by solvent on NH3BH3 giving a solvent-borane adduct, and (3) reaction with the solvent leading to products consistent with a hydroboration pathway. The etheral solvents glyme, diglyme, tetrahydrofuran and 2-methyltetrahydrofuran gave mainly products suggesting hydrogen-loss decomposition of NH3BH3.
Nanophase Ammonia Borane in Mesoporous SBA-15
Gutowska reported the preparation of nanophase AB by incorporating AB into the channels of mesoporous silica SBA-15 (Figure 4).20 This resulted in significantly different behavior lowering the dehydrogenation temperature from 114 °C to 85 °C accompanied by a relatively low exothermicity. The two other notable effects resulting from the nanostructure of AB in the SBA-15 scaffold include: 1) the temperature threshold for H2 release (m/e=2) being notably lower than the temperature threshold for neat AB indicative of an enhanced rate of H2 release; and 2) the yield of the borazine side product (m/e=80) is significantly lower than for neat AB. Figure 5 shows the effect of mesoporous silica encapsulation on thermodynamics of ammonia borane. Solid-state11 B NMR spectroscopy was used to analyze the nonvolatile products from neat AB and AB:SBA-15 to see if the borazine became entrapped within the mesoporous scaffold. However, no11 B signal for borazine was observed. Because borazine is not observed as a volatile product in the TPD/MS experiment, and not detected by solid-state NMR spectroscopy, the mesoporous scaffold appears to affect the decomposition pathways of AB that lead to hydrogen formation.
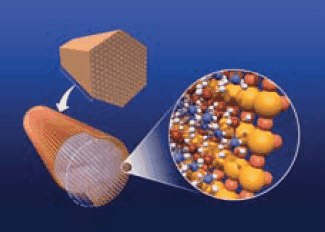
Figure 4. Illustration depicting incorporation of ammonia-borane into the channels of mesoporous silica SBA-15.
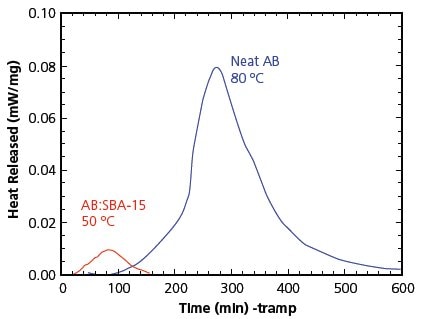
Figure 5. Isothermal DSC comparisons showing lower reaction enthalpy for H2 loss from AB on mesoporous silica SBA-15.
Summary
While ammonia borane looks promising for hydrogen storage, given the volumetric and gravimetric density of hydrogen in the material, there are still technical challenges to be addressed. Foremost of these challenges are (1) enhancing the rates of hydrogen release and (2) the discovery of economical chemical processing pathways that will be used to put the hydrogen back on to the dehydrogenated materials. Our group is currently focusing on these technical challenges.
Acknowledgments
Support for this work by the U.S. Department of Energy, Energy Efficiency and Renewable Energy, and Office of Science, Basic Energy Sciences, Chemical Sciences Division is gratefully acknowledged. The Pacific Northwest National Laboratory is operated by Battelle for the US DOE.
References
如要继续阅读,请登录或创建帐户。
暂无帐户?