Drug transport assays in a 96-well system: reproducibility and correlation to human absorption
Background
Successful development of new pharmaceuticals must include testing of their Absorption, Distribution, Metabolism, Excretion and Toxicity (ADMET) properties to ensure that they are suitable for oral administration. Absorption is defined as a drug’s ability to cross epithelial and endothelial cell barriers from the point of administration to the site of action. This absorption can occur either through passive transcellular or paracellular diffusion, active carrier transport, or active efflux mechanisms. Several methods have been developed to aid in the under-standing of the absorption of a chemical compound. The most common ones use an immortalized cell line (e.g., Caco-2, MDCK, etc.) to mimic the intestinal epithelium. These in vitro models provide more predictive permeability information than artificial membrane systems (i.e., PAMPA and Permeability assays) based on the cells’ ability to promote (active transport) or resist (efflux) transport.1,2
The Caco-2 cell line is heterogeneous and was derived from a human colorectal adenocarcinoma.3 Caco-2 cells are used as in vitro permeability models to predict human intestinal absorption because they exhibit many features of absorptive intestinal cells.4-6 This includes their ability to spontaneously differentiate into polarized enterocytes that express high levels of brush border hydrolases and form well developed junctional complexes (see Figure 1, cross-section electron micrograph (EM) of differentiated Caco-2 cells).7 Consequently, it becomes possible to determine whether passage is transcellular or paracellular based on a compound’s transport rate. Caco-2 cells also express a variety of transport systems including di-peptide transporters and P-glycoproteins (Pgp). Due to these features, drug permeability in Caco-2 cells correlates well with human oral absorption, making Caco-2 an ideal in vitro permeability model.8, 9 Additional information can be gained on metabolism and potential drug-drug interactions as the drug undergoes transcellular diffusion through the Caco-2 transport model.10
Although accurate and well researched, the Caco-2 cell model requires a high investment of time and resources. Depending on a number of factors, including initial seeding density, culturing conditions, and passage number, Caco-2 cells can take as much as 20 days to reach confluence and achieve full differentiation. During this 20-day period, they require manual or automated exchange of media as frequently as every other day. The transport assays consume valuable drug compounds and normally require expensive, post transport sample analyses (e.g. LC/MS). Therefore, the use of the Caco-2 transport model in a high throughput laboratory setting is only possible if the platform is robust, automation compatible, reproducible, and provides high quality data that correlate well with established methodologies.
Introduction
The Millipore Millicell-96 system is a reliable 96-well platform for predicting human oral absorption of drug compounds (using Caco-2 cells or other cell lines whose drug transport properties have been well characterized). The system format is automation compatible and is designed to offer more cost effective, higher throughput screening of drugs than a 24-well system. The Millicell-96 system exhibits good uniformity of cell growth and drug permeability across all 96 wells and low variability between production lots. The plate design supports the use of lower volumes of expensive media and reduced amounts of test compounds. Using the Millicell-96 system, standard drug compounds are successfully categorized as either "high" or "low" permeable, as defined by the
Food and Drug Administration, and the permeability data correlate well with established human absorption values.11
The data presented in this Application Note demonstrates that drug permeability rates obtained using Millicell-96 system correlate well with published human intestinal absorption values. Additionally, there is uniform performance within plates, when comparing one plate to another and when testing multiple plate lots. Correlation to human absorption was investigated directly through experiments done independently by ArQule, Inc.12
These results, encompassing testing of an extensive list of compounds, were originally presented in a poster at the 2002 AAPS Annual Meeting and Exposition in Toronto, Canada. Experiments were also performed comparing drug permeability rates measured with the Millicell-96 system to those measured using a 24-well system. The goal of this research was to determine whether data obtained with the Millicell-96 (i.e., a 96-well platform) would be comparable to permeability results obtained using an established system and thus, indirectly, provide additional correlation to percent human absorption. Finally, product variability was explored by testing the transport of propranolol in all 96 wells on multiple Millicell-96 chosen from different production lots and seeded with cells on different days.
Figure 1 (left): Cross-sectional EM Figure 2 (right): Millicell-96 Differentiated Caco-2 Cells
Components:
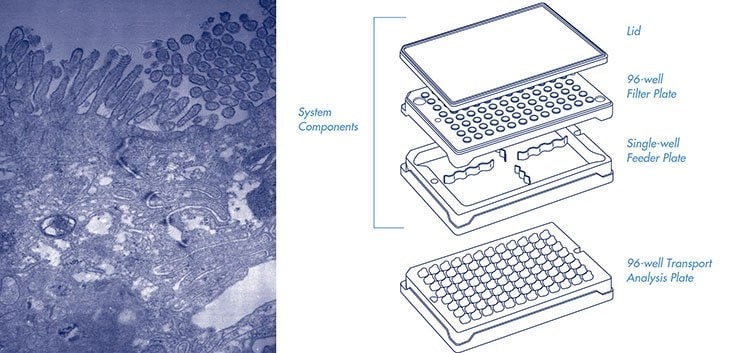
Millicell-96 assay system components (cat. PSHT004R5, with single-well
feeder plate) and 96-well transport analysis plate (cat. MACA C0R S5). Not shown,
assay system components with 96-well feeder plate (cat. PSHT004S5).
Materials and Equipment
Tissue Culture
Non-essential amino acids (cat. M7145), HEPES (cat. H0887),penicillin, streptomycin and L-glutamine (cat. G1146), EDTA (cat. E8008) trypsin/EDTA (cat. T3924), and the multiwall plate washer, 8-channel manifold (cat. M2656) were all purchased from Sigma Chemical Co. (St. Louis, MO). Fetal bovine serum (cat. 16000044) and Hank’s balanced salt solution (HBSS) (cat. 14025) were purchased from Invitrogen (Carlsbad, CA). Millicell-96 system (cat. PSHT004S5 or PSHT004R5) was purchased from Millipore Corporation (Bedford, MA). Corning HTS Transwell®‚ -24 plates and reservoirs (cat. 07200687 and 08774162) and tissue culture flasks (cat. 1368065) were purchased from Fisher Scientific.
Electrical Resistance Measurements Millicell®-ERS-2 probe for the 24-well plates (cat. MERSSTX01) and a Millicell-ERS ohm meter (cat. MERS00002) were obtained from Millipore Corporation. An electrode for measuring electrical resistance in the Millipore plate (cat. STX 100M) was purchased from World Precision Instruments (Sarasota, FL).
Drug Transport Experiments
Propranolol (cat. P0884), testosterone (cat. T1500), digoxin (cat. D6003), mannitol (cat. M9546), paclitaxel (Taxol®) (cat. T1912), prednisone (cat. P6254), ibuprofen (cat. I1892), verapamil (cat. V4629), warfarin (cat. A2250) and methotrexate (cat. M8407) were purchased from Sigma Chemical Co. (St. Louis, MO). Radio- labeled mannitol (NET101), digoxin (NET222), propranolol (NET515), testosterone (NET553) and verapamil (NET810) were purchased from PerkinElmer (Boston, MA). Radio- labeled Taxol (MT-552), methotrexate (MT-701) and warfarin (MT-1620) were purchased from Moravek (Brea, CA). Radio-labeled ibuprofen (ART-392) and prednisone (ART-836) were purchased from ARC (St. Louis, MO). Wallac 1450 Microbeta® Plus Scintillation Counter, Microbeta Trilux Multiwell Plate Scintillation Counter and Microbeta 96-well flexible plates (1450-401) were purchased from Wallac/PerkinElmer (Boston, MA).
Methods
Performing Drug Transport Assays
For specific methods concerning Cultivation and Feeding of Caco-2 Cells, Optimizing Cell Seeding Density for the Millicell-96 system, and Performing a Drug Transport Assay using the Millicell-96 system, see Millipore Protocol Note PC1060EN00: Optimization of Caco-2 cell growth and differentiation for drug transport assay studies using a 96-well assay system.13
In these experiments, Caco-2 cells were cultivated in tissue culture flasks to 80-90% confluence. Cells at pass- ages 33-35 were used to seed one MultiScreen Caco-2 plate (96-well filter plate from cat. PSHT004S5 or PSHT004R1) and four 24-well plates for 21-day cell growth in four independent experiments (each experiment was performed on a different day). The optimal seeding density was determined to be 82,000 cells per cm2 for the Millicell-96, and 67,000 cells per cm2 for the 24-well plates. All plates were fed apically and basolaterally every other day.
The transepithelial electrical resistance (TEER) was measured in each well after 21 days. (Wells that registered TEER values below 100 ohms x cm2 did not meet previously established acceptance criteria for proper cell monolayer formation and the data from these wells were not incorporated into the graphs.) The plates were subsequently washed three times with sterile HBSS, pH 7.4. Ten tritium-labeled compounds were diluted 100-fold into solutions containing their respective unlabeled counterparts. This mixture was brought to a final concentration of 10 µM in HBSS, pH 7.4 and then, added apically to all 96 wells of each plate. Drug transport was determined after two hours (shaking at 37 °C, 5% CO2, 95% relative humidity) using a Trilux Multiwell Plate Scintillation Counter to measure counts per minute of radio-labeled drug. Drug perme- ability values were then calculated using the equation described in the next section. For detailed methods used by ArQule, Inc. see reference.12 Briefly, cells between passage numbers 18–25 were used in these experiments. Millicell-96 plates were seeded at a density of 65,000 cells per cm2 and the cells were grown for 21–28 days. Permeability of non-radio-labeled compounds (20 µM) was measured after two hours, with shaking at 37 °C. Samples were analyzed using liquid chromatography/mass spectrometry. Permeability of radio- labeled compounds (trace amounts mixed with 1 µM of their respective unlabeled counterparts) was measured at different time points over 80 minutes. These samples were quantified using liquid scintillation counting.
Evaluating the Reproducibility of Drug Transport Experiments
For these experiments, cells at passage 33 and 34 were used to seed plates from three different lots of PSHT004S5 and one lot of PSHT004R5for 21-day cell growth in three independent experiments (each experiment was performed on a different day). The seeding density and performance of TEER evaluation and drug transport assays are described above.
Results
Evaluation of Monolayer Integrity
Prior to the start of all drug transport experiments, the TEER was measured in every well to confirm monolayer integrity. The TEER measurements for all experiments discussed in this section were typically in the range of 200 to 350 ohms x cm2 (data not shown). This range is indicative of successful formation of integral monolayers after 21 days of culture. The corresponding drug transport data confirm differentiated monolayers with tight junctions.
Correlation of Drug Transport Rates to Human Absorption
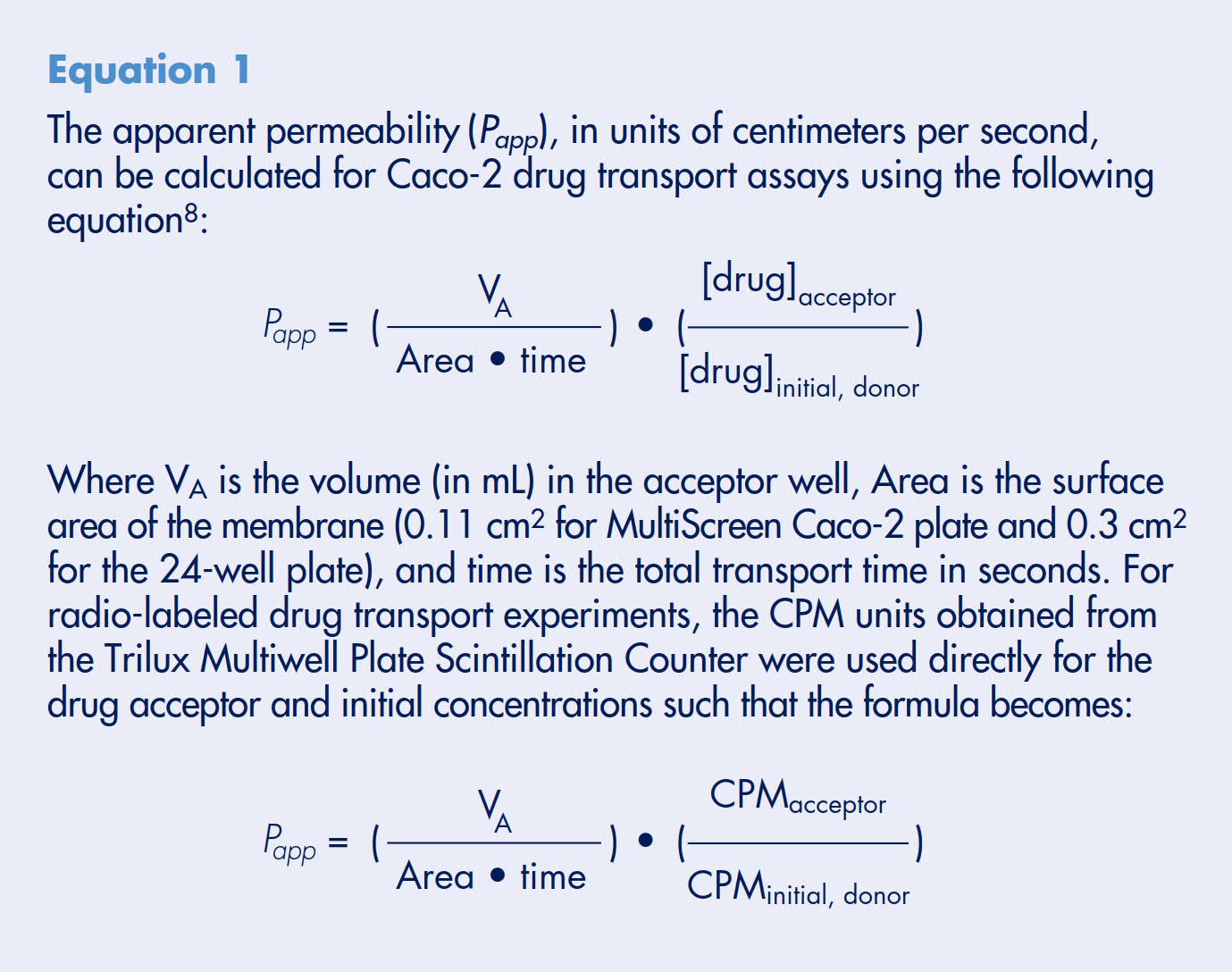
Historically, it has been shown that a sigmoidal relationship exists between drug absorption rates as measured with the in vitro Caco-2 model and human absorption.8 Drug compounds representing active, passive, and efflux transporters were tested at ArQule, Inc. using the Millicell-96 system.12 Each compound’s permeability rate was plotted against their percent human absorption values (Figure 3). The passive diffusion compounds included in this evaluation were carbamazepine, mannitol, penicillin, caffeine, buspirone, metoclopramide, haloperidol, prometh- azine, and imipramine. The active influx compounds were methotrexate, piroxicam, salicylic acid, and Gly-Sar. The efflux compounds included were loperamide, clomipramine, metolazone, alprenolol, timolol, metoprolol, digoxin, chlorpromazine, quinine, paclitaxel, thiacetazone, acebutolol, sulfasalazine, doxorubicin, and thioridazine. Using Statistica™ (Stat Soft Corporation, Tulsa, OK), a nonlinear regression curve, Equation 2, was generated and provided a good fit to all of the data points. Statistical analysis of the curve suggested that there were no significant differences in fit based on whether the drug was absorbed actively, passively or effluxed (ANOVA F-test on single curve ranked residuals; p=0.33). Based on the curve fit, transport rates were typically > 1.0 x 10-5 cm/s and < 1.0 x 10-6 for high and low permeable compounds, respectively. Intermediate compounds were estimated to have an upper limit of permeability of ~7.0 x 10–6. This study demonstrated that the Millicell-96 system could correctly identify and classify the permeability properties of all 25 compounds tested.
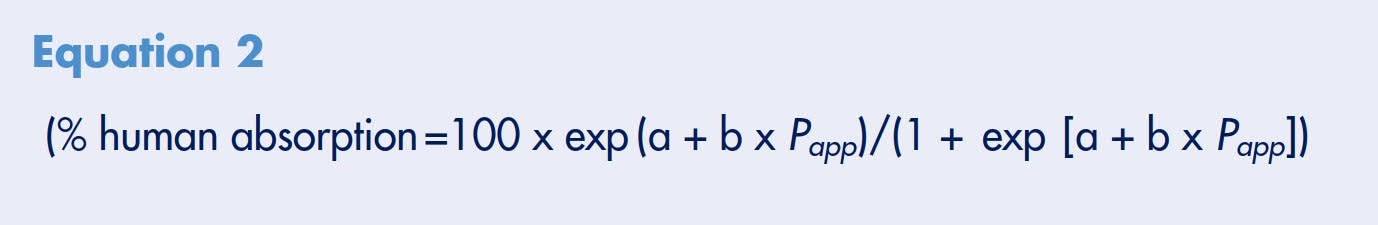
Figure 3: Correlation of Drug Transport Rates to Human Absorption*
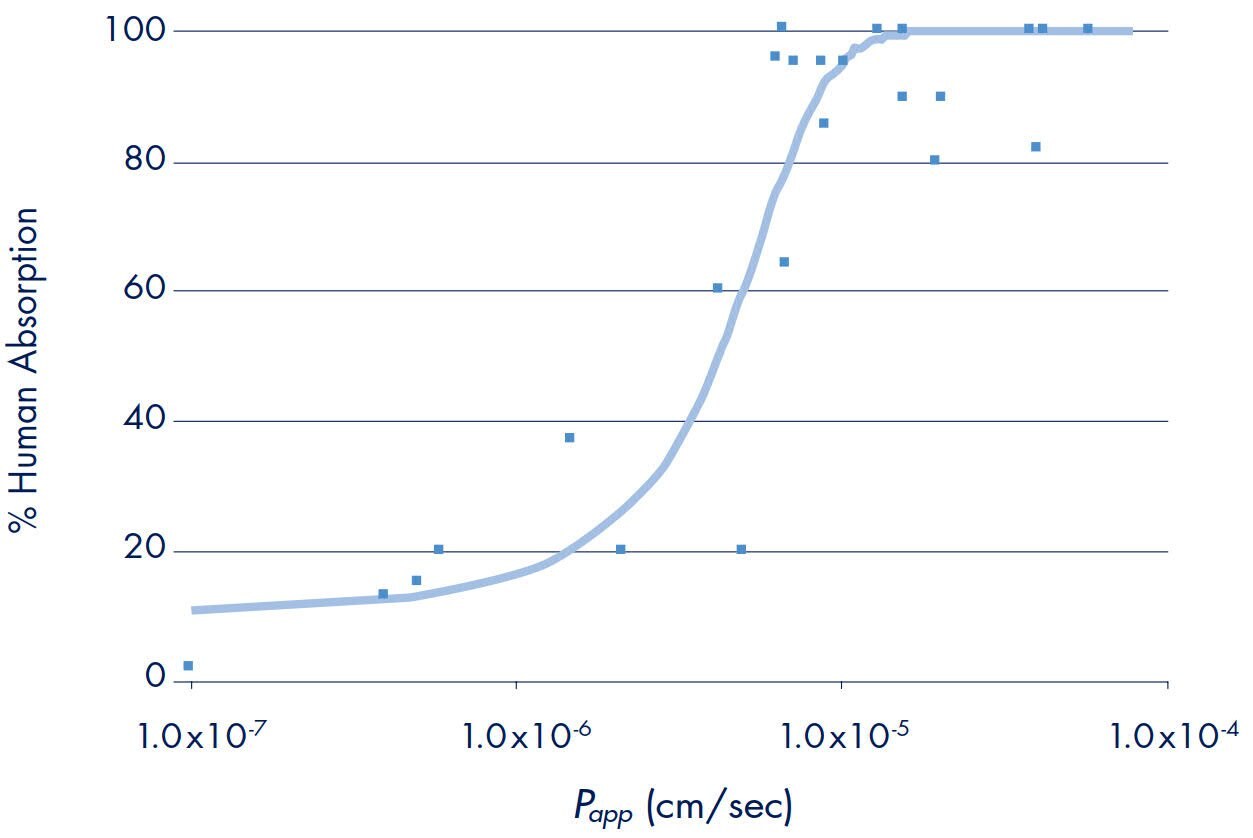
*One outlier, terfenadine, which had a Papp of 1x10-7 cm/s and % human abs =100%, was not included.
Correlation of Drug Transport
Rates to 24-well Plate Results
A significant amount of literature supports the use of a 24-well system for in vitro drug permeability models. When developing or validating a 96-well platform, it is important to correlate data to results obtained using previously established methods. Experiments using the same cells and methods were performed to compare the drug transport rates obtained from the Millicell-96 and an established 24-well system. Caco-2 permeability rates using 10 tritium labeled drugs (mannitol, digoxin, propranolol, testosterone, Taxol, prednisone, verapamil, warfarin, ibuprofen, and methotrexate) were measured using the Millicell-96 or 24-well assay system. Data from four separate experiments are compared in Figure 4. The R2 value of 0.99 suggests that the data between the two different formats (i.e., 24-well and 96-well) correlate well. Further evidence of good correlation is demonstrated when the transport rates (excluding digoxin and Taxol) are plotted against human absorption values (Figure 5). The same curve generated in Figure 3 adequately fits both sets of data. Thus, when adopting the Millicell-96 system for in vitro permeability studies, it is expected that the data will correlate to a 24-well system.
Figure 4. Comparison of Permeability Rates: Millicell-96 and 24-well Assay Systems
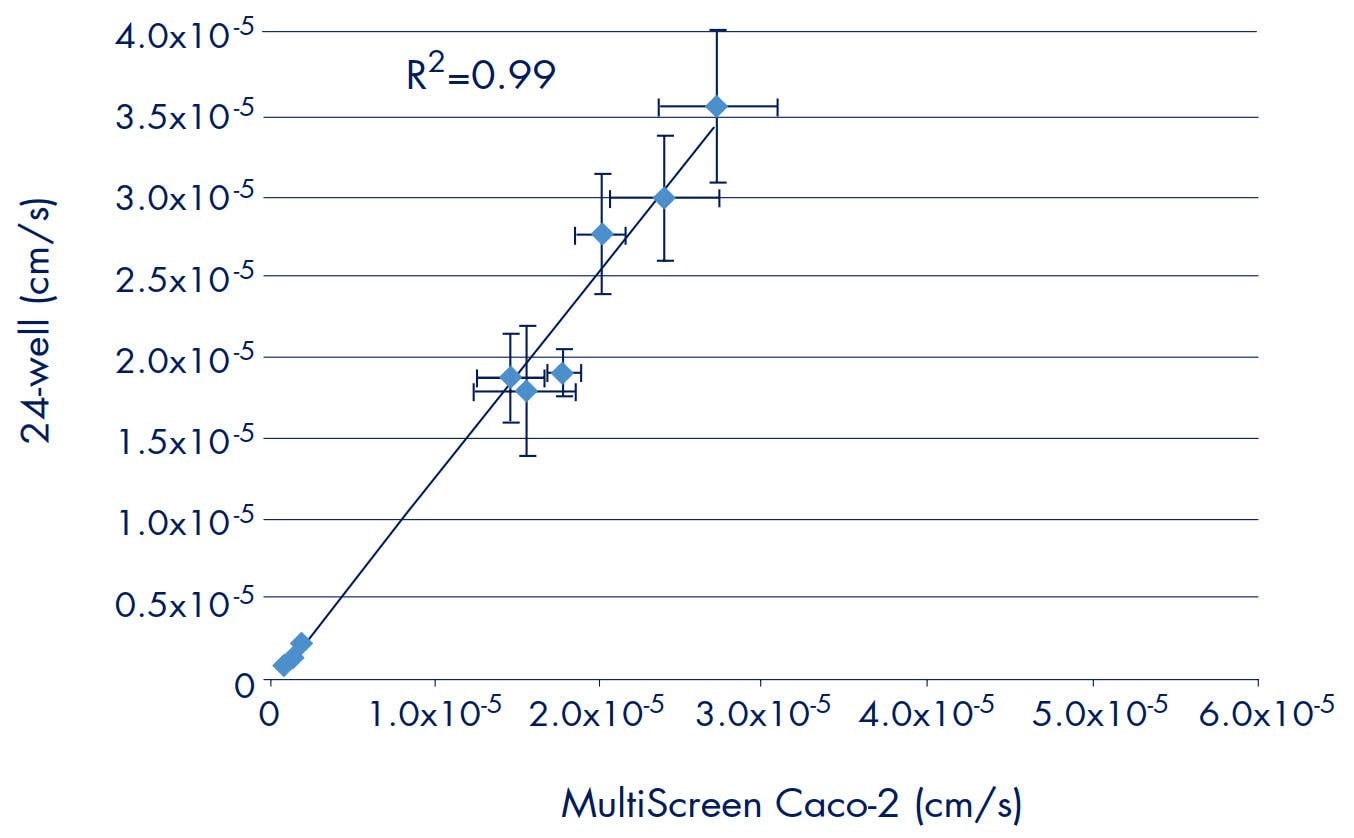
Note: The average transport rates used to create Figures 4 and 5 do not include any replicates that were obvious outliers. This was seen in more than one experiment and occurred in both products.
Figure 5, Papp vs. Human Absorption—Comparison of Millicell-96 and 24-well Assay Systems
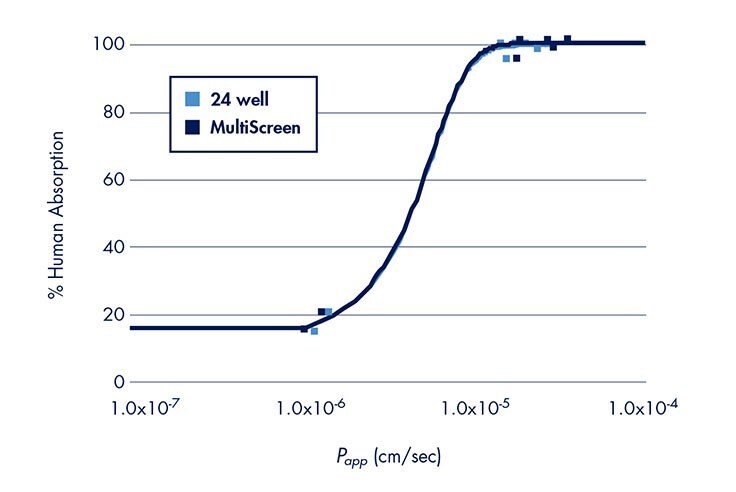
Reproducibility of Drug Transport Rates
The reliability of cell-based in vitro methods is contingent upon minimizing the variability contributed by the cell growth platform. The use of multi-well plates for growing cells in long-term cultures may present a challenge from both an environmental and industrial perspective. During culture, even in a humidity-controlled incubator, elevated temperatures can cause evaporation to occur more rapidly in the outer wells of the plate as compared to the inner wells. Minor differences in the seeding of plates from day to day may also contribute to variability. Based on these factors, reproducibility of drug transport rates was examined in the Millicell-96 system using a 21-day culture of Caco-2 cells and a single drug, propranolol, in all 96 wells of every plate tested. Transport rates were determined in the apical to basolateral direction. The components of variation were calculated from the data set consisting of three experimental runs performed on different days. Each run consisted of four different production lots of Millicell-96 plates, three lots with 96-well feeder trays (feeder tray from cat. MACA CO2 S5), and one lot with a single-well feeder tray (feeder tray from cat. MACA CO2 B5). The intra-plate comparison of propranolol transport in the outer 36 wells versus the inner 60 wells was plotted for all 12 plates in this study (figure not shown). The resultant R2=0.9 confirms that the average value and variability of the external wells (n=36) was statistically similar to the average value and variability of the internal wells (n=60).
Analysis of variance on the plate averages, inter-plate and intra-plate standard deviations (SD) was determined for all 12 plates. The overall plate average permeability rate for these experiments is 1.5 x 10-5 cm/sec. The intra-plate and inter-plate standard deviations are estimated at 0.2 x10-5 cm/sec and do not vary significantly among the production lots or test days (p>0.07 and p>0.17, respectively). Using these values, the total standard deviation (total SD) of a single measurement is calculated to be 0.3 x10-5 cm/sec using Equation 3
Therefore, 95% of all results from a propranolol transport experiment, using the Millicell-96 system, are predicted to fall within the range determined using Equation 4. This predicts that 95% of all propranolol transport rates (apical to basolateral) in this study should fall within the range of 0.9 to 2.1 x 10-5 cm/sec. These experiments also demonstrate that the confidence range should be achieved independent of well position, plate production lot, type of feeding tray used, or day of experiment (Figure 6). A line representing the upper limit of low permeability compounds (extrapolated from Figure 3) is drawn to demonstrate that the 95% confidence range clearly distinguishes propranolol as a high versus a low permeable compound.
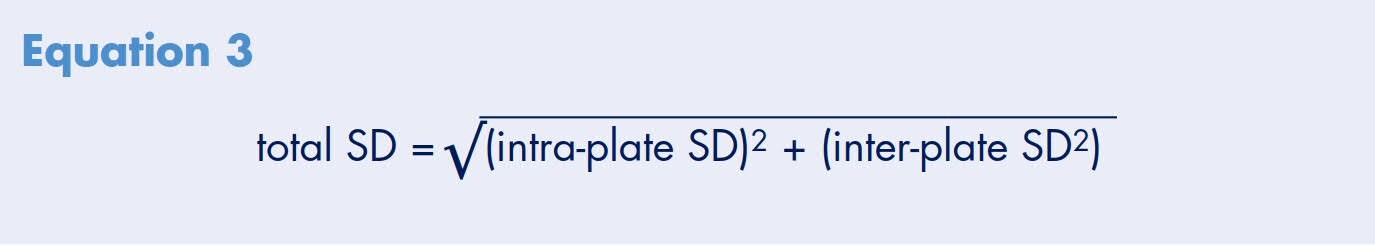

Figure 6. Inter-plate Variation of Propranolol Transport*
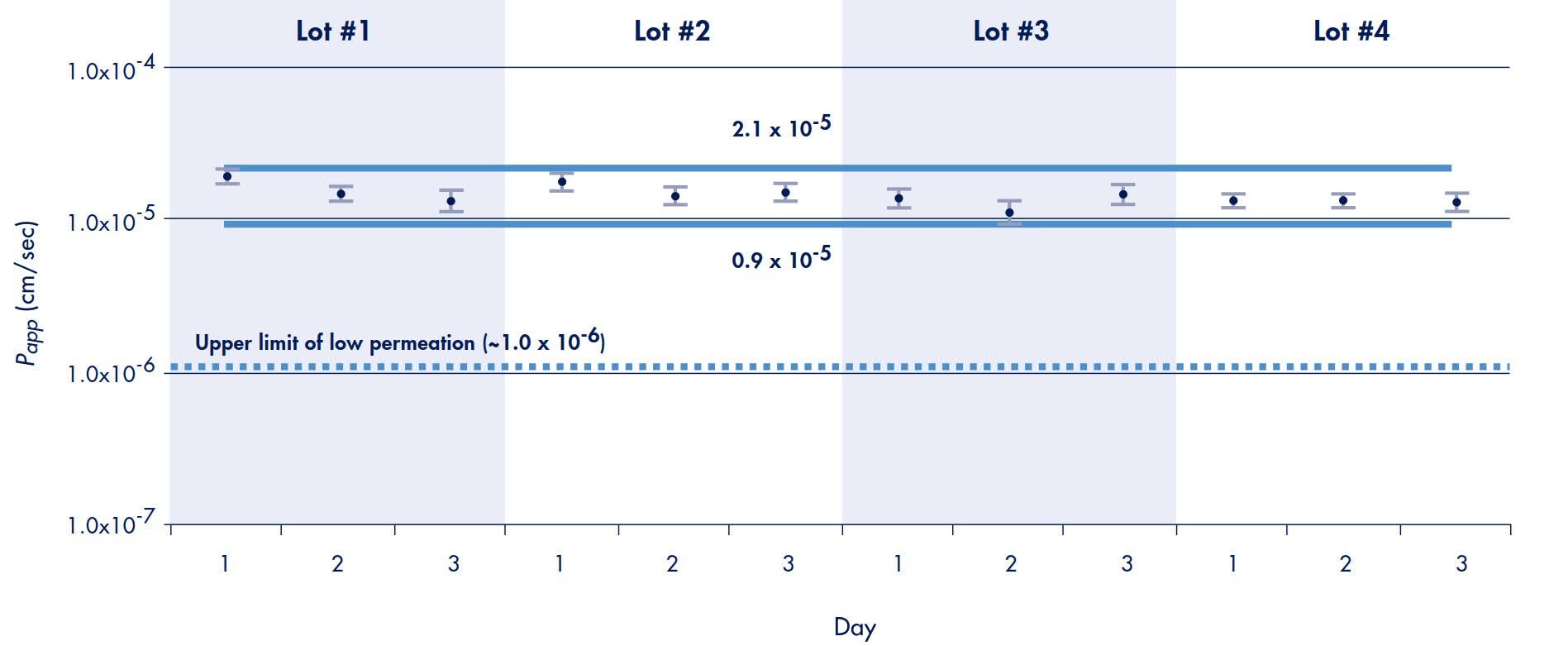
*Note: Each data point represents a single plate and the average and standard deviation of 96 wells
Discussion
Caco-2 cells are heterogeneous and their properties in final culture may differ based on the selection pressures of a particular laboratory. Direct comparison of compound permeability rates between laboratories is not possible unless the same Caco-2 cells and conditions are used.14 Therefore, transport rates and permeability classification ranges of specific drugs are expected to vary from data presented in this Application Note. Most important is the ability to successfully classify compounds as low, medium or high permeable drugs and produce transport results that correlate to established human absorption values. The Multi-Screen Caco-2 assay system satisfies these requirements as evidenced by the data presented in Figure 3. Extrapolations from that curve produced ranges that correctly classified all compounds based on permeability. The comparison studies with the 24-well system yielded a high degree of correlation. This similarity to established systems demonstrates that the MultiScreen Caco-2 system can be used in place of 24-well systems as a platform for increasing the throughput and/or automating in vitro permeability screening assays. Although inter-laboratory variability is likely to remain an issue when using Caco-2 cell methods, the Millicell-96 system will provide low variability within a laboratory along with correct compound classification (Figure 6).
Conclusion
The results presented in this Application Note demonstrate that the Millicell-96 system provides a robust and reproducible method for predicting human absorption of drug-like compounds. The plates — which are compatible with a wide range of laboratory robotics — are designed to grow stabile, differentiated monolayers and provide reliable and accurate Caco-2 permeability data.
Materials
如要继续阅读,请登录或创建帐户。
暂无帐户?