四唑盐法(MTT)细胞活力和增殖检测方案
四唑盐(MTT)法通过检测细胞代谢活性指示细胞存活、生长增殖和毒性状态。这种比色法的原理是代谢活性细胞可将黄色的四唑盐(3-(4,5-二甲基-2-噻唑基)-2,5-二苯基溴化四唑或MTT)还原为紫色的甲臜(Formazan)结晶物(图1)。6,7,35活细胞中的NAD(P)H依赖性氧化还原酶负责将MTT还原为甲臜。36用溶解液溶解不溶性甲臜结晶物,可在微孔板分光光度计下测定溶液在500-600纳米波长处的光吸收值,实现定量。溶液颜色越深,代表具有代谢活性的活细胞数目越多。
这种非放射性的MTT比色法由Mosmann, T等人首先提出,1其后经过多名研究人员改良。2-6MTT细胞增殖检测试剂盒I是优化的MTT测定试剂盒,由即用型试剂组成,无需洗涤步骤或额外试剂。可快速方便地检测大量样品。MTT细胞增殖检测试剂盒适合多种应用,包括:
- 细胞生长和活力的定量分析。1,3,5-7
- 检测生长因子、细胞因子和营养物质对细胞增殖的影响。1-3,6,8-12(见图3)。
- 细胞毒性检测。例如,定量分析肿瘤坏死因子a或b的细胞毒性作用13,14(图2)或巨噬细胞诱导的细胞死亡15,16,评估细胞毒性 17-34或生长抑制剂作用,如抑制性抗体。
- 细胞激活研究。4
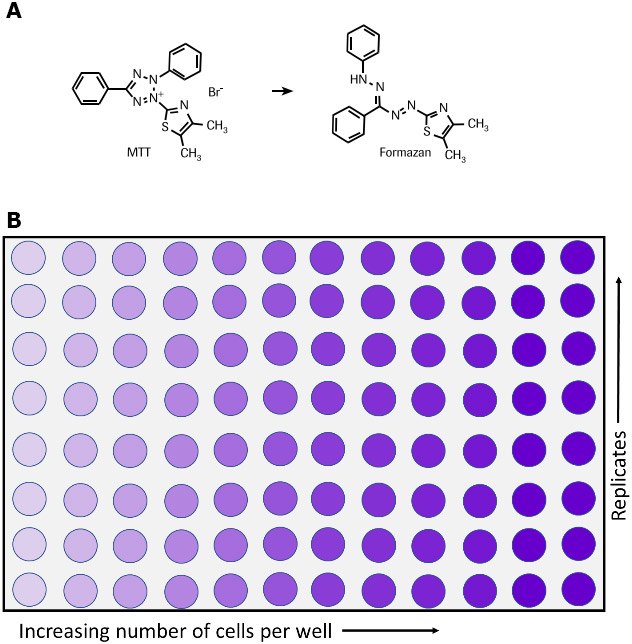
图 1.活细胞将MTT转化为甲臜的代谢反应,(A)反应式,(B)96孔板检测。
MTT细胞增殖检测试剂盒I组分(产品编号11465007001)
MTT试剂
- 即用型,非无菌
- 5瓶,每瓶5 ml MTT(3-(4,5-二甲基-2-噻唑基)-2,5-二苯基溴化四唑)(1X),5 mg/mL,溶于磷酸盐缓冲液(PBS,806552)
溶解液
- 1X,即用型
- 3瓶,每瓶90 mL
细胞毒性检测方案
自备试剂:
- 培养基,例如RPMI 1640培养基(R0883),添加10%热灭活FCS(胎牛血清,12106C),2 mM谷氨酰胺(G6392)和1 μg/mL放线菌素C1(放线菌素D,A9415)。
- 如用到抗生素,在培养基中再添加青霉素/链霉素或庆大霉素
- 人肿瘤坏死因子-α(hTNF-α)(10 μg/mL),无菌(T6674)。
步骤:
用于测定人肿瘤坏死因子-α(hTNF-α, T6674)对WEHI-164细胞(小鼠纤维肉瘤,87022501)的毒性作用(见图2)。
- 用培养基(含1 μg/mL放线菌素C1)将WEHI-164细胞配成1 × 106 cells/ml浓度,在37 °C和5-6.5% CO2下先培养3 h。
- 将细胞接种到微孔板(组织培养级,96孔,平底),接种密度5 × 104 cells/孔,100 μl培养基,其中含1 μg/mL放线菌素C1和不同含量的hTNF-α(例如,终浓度0.001–0.5 ng/mL)。
- +37 °C和5-6.5% CO2下培养24 h。
- 培养后,每孔加入10 μl MTT标记试剂(终浓度0.5 mg/ml)。
- 将微孔板放在加湿环境中(例如,+37 °C, 5-6.5% CO2)孵育4 h。
- 每孔加入100μl溶解液。
- 微孔板置于培养箱,在加湿条件下(例如,+37 °C, 5-6.5% CO2)孵育过夜。
- 确保紫色甲臜结晶物充分溶解,在微孔板酶标仪中测定样品吸光度值。根据所用酶标仪的滤光片配置,选择550至600 nm间的某处波长测定甲臜产物的吸光度值。参比波长(reference wavelength)应超过650 nm。
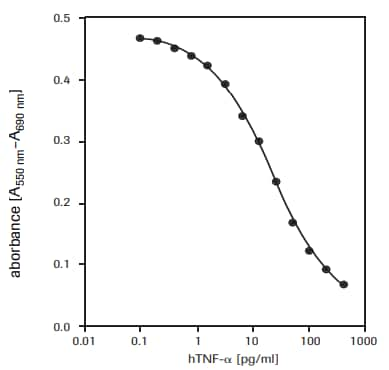
图 2.重组人肿瘤坏死因子-α(hTNF-α)对WEHI-164细胞(小鼠纤维肉瘤,87022501)的毒性作用检测。
细胞生长检测方案
自备试剂
- 培养基(例如,DMEM,产品编号D5671),添加10%热灭活FCS(胎牛血清,产品编号12106C)
- 2 mM谷氨酰胺(产品编号G6392)
- 0.55 mM L-精氨酸(产品编号A8094)
- 0.24 mM L-天冬酰胺一水物(产品编号A4284)
- 50 μM 2-巯基乙醇(产品编号M3148),
- HT培养基补充物(产品编号H0137)(1×),含0.1 mM次黄嘌呤和16 μM胸苷。如用到抗生素,在培养基中再添加青霉素/链霉素或庆大霉素。
- 人白细胞介素-6(hIL-6,产品编号SRP3096)(200,000 U/mL, 2 μg/mL) ,无菌
步骤
用于测定人白介素-6(hIL-6)对7TD1细胞(鼠-鼠杂交瘤细胞,DSMZ,ACC 23)的增殖刺激作用(见图3)。
- 将7TD1细胞接种到微孔板(组织培养级,96孔,平底),接种密度2 × 103 cells/孔,100 μl培养基,其中含不同含量的IL-6【例如,终浓度0.1-10 U/mL (0.001-0.1 ng/mL)】。
- +37 °C和5-6.5% CO2下培养4天。
- 培养后,每孔加入10 μl MTT标记试剂(终浓度0.5 mg/ml)。
- 将微孔板放在加湿环境中(例如,+37 °C, 5-6.5% CO2)孵育4 h。
- 每孔加入100 μL溶解液。
- 微孔板置于培养箱,在加湿条件下(例如,+37 °C, 5-6.5% CO2)孵育过夜。
- 确保紫色甲臜结晶物充分溶解,在微孔板酶标仪中测定样品吸光度值。根据所用酶标仪的滤光片配置,选择550至600 nm间的某处波长测定甲臜产物的吸光度值。参比波长(reference wavelength)应超过650 nm。
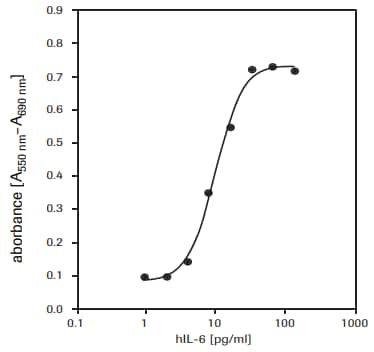
图 3.MTT法检测人重组白介素-6(hIL-6)对7TD1细胞(鼠-鼠杂交瘤细胞)的增殖影响。
相似检测
XTT法和WST-1法也可用于细胞活力和生长增殖的检测。
产品
Loading
参考文献
References 1-20
1.
Mosmann T. 1983. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. Journal of Immunological Methods. 65(1-2):55-63. https://doi.org/10.1016/0022-1759(83)90303-4
2.
Tada H, Shiho O, Kuroshima K, Koyama M, Tsukamoto K. 1986. An improved colorimetric assay for interleukin 2. Journal of Immunological Methods. 93(2):157-165. https://doi.org/10.1016/0022-1759(86)90183-3
3.
Denizot F, Lang R. 1986. Rapid colorimetric assay for cell growth and survival. Journal of Immunological Methods. 89(2):271-277. https://doi.org/10.1016/0022-1759(86)90368-6
4.
Gerlier D, Thomasset N. 1986. Use of MTT colorimetric assay to measure cell activation. Journal of Immunological Methods. 94(1-2):57-63. https://doi.org/10.1016/0022-1759(86)90215-2
5.
Hansen MB, Nielsen SE, Berg K. 1989. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. Journal of Immunological Methods. 119(2):203-210. https://doi.org/10.1016/0022-1759(89)90397-9
6.
Vistica VT, Skehan P, Scudiero D, Monks A, Pittman A, Boyd MR. 1991. Tetrazolium-based assays for cellular viability: a critical examination of selected parameters affecting formazan production. Cancer Res. 51(10):2515-20.
7.
Maehara Y, Anai H, Tamada R, Sugimachi K. 1987. The ATP assay is more sensitive than the succinate dehydrogenase inhibition test for predicting cell viability. European Journal of Cancer and Clinical Oncology. 23(3):273-276. https://doi.org/10.1016/0277-5379(87)90070-8
8.
Heeg K, Reimann J, Kabelitz D, Hardt C, Wagner H. 1985. A rapid colorimetric assay for the determination of IL-2-producing helper T cell frequencies. Journal of Immunological Methods. 77(2):237-246. https://doi.org/10.1016/0022-1759(85)90036-5
9.
Hooton, Gibbs J, Paetkan U C. 1985. Interaction of interleukin 2 with cells: quantitative analysis of effects.. J Immunol. 1352464–2473.
10.
Mosmann T, Fong T. 1989. Specific assays for cytokine production by T cells. Journal of Immunological Methods. 116(2):151-158. https://doi.org/10.1016/0022-1759(89)90198-1
11.
Ohno M, Abe T. 1991. Rapid colorimetric assay for the quantification of leukemia inhibitory factor (LIF) and interleukin-6 (IL-6). Journal of Immunological Methods. 145(1-2):199-203. https://doi.org/10.1016/0022-1759(91)90327-c
12.
Berg K, Hansen MB, Nielsen SE. 1988. J. Interferon Res.. 8((suppl. 1)):S. 67.
13.
Green LM, Reade JL, Ware CF. 1984. Rapid colormetric assay for cell viability: Application to the quantitation of cytotoxic and growth inhibitory lymphokines. Journal of Immunological Methods. 70(2):257-268. https://doi.org/10.1016/0022-1759(84)90190-x
14.
Espevik T, Nissen-Meyer J. 1986. A highly sensitive cell line, WEHI 164 clone 13, for measuring cytotoxic factor/tumor necrosis factor from human monocytes. Journal of Immunological Methods. 95(1):99-105. https://doi.org/10.1016/0022-1759(86)90322-4
15.
Ferrari M, Fornasiero MC, Isetta AM. 1990. MTT colorimetric assay for testing macrophage cytotoxic activity in vitro. Journal of Immunological Methods. 131(2):165-172. https://doi.org/10.1016/0022-1759(90)90187-z
16.
van de Loosdrecht AA, Nennie E, Ossenkoppele GJ, Beelen RH, Langenhuijsen MM. 1991. Cell mediated cytotoxicity against U 937 cells by human monocytes and macrophages in a modified colorimetric MTT assay. Journal of Immunological Methods. 141(1):15-22. https://doi.org/10.1016/0022-1759(91)90205-t
17.
Cole S. 1986. Rapid chemosensitivity testing of human lung tumor cells using the MTT assay. Cancer Chemother. Pharmacol.. 17(3): https://doi.org/10.1007/bf00256695
18.
Carmichael J, DeGraff WG, Gazdar AF, Minna JD, Mitchell JB. 1987. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res. 47(4):936-42.
19.
Twentyman P, Luscombe M. 1987. A study of some variables in a tetrazolium dye (MTT) based assay for cell growth and chemosensitivity. Br J Cancer. 56(3):279-285. https://doi.org/10.1038/bjc.1987.190
20.
Park JG, Park BS, Steinberg SM, Carmichael J, Collins JM, Minna JD, Gazdar AF. 1987. Chemosensitivity testing of human colorectal carcinoma cell lines using a tetrazolium-based colorimetric assay. Cancer Res. 475875-9.
References 21-36
21.
22.
Pieters R, Huismans D, Leyva A, Veerman A. 1988. Adaptation of the rapid automated tetrazolium dye based (MTT) assay for chemosensitivity testing in childhood leukemia. Cancer Letters. 41(3):323-332. https://doi.org/10.1016/0304-3835(88)90294-7
23.
McHaLe A, McHaLe L. 1988. Use of a tetrazolium based colorimetric assay in assessing photoradiation therapy in vitro. Cancer Letters. 41(3):315-321. https://doi.org/10.1016/0304-3835(88)90293-5
24.
Edmondson JM, Armstrong LS, Martinez AO. 1988. A rapid and simple MTT-based spectrophotometric assay for determining drug sensitivity in monolayer cultures. Journal of Tissue Culture Methods. 11(1):15-17. https://doi.org/10.1007/bf01404408
25.
Faircloth GT, Stewart D, Clement JJ. 1988. A simple screening procedure for the quantitative measurement of cytotoxicity to resting primary lymphocyte cultures. Journal of Tissue Culture Methods. 11(4):201-205. https://doi.org/10.1007/bf01407314
26.
Campling BG, Pym J, Galbraith PR, Cole SP. 1988. Use of the mtt assay for rapid determination of chemosensitivity of human leukemic blast cells. Leukemia Research. 12(10):823-831. https://doi.org/10.1016/0145-2126(88)90036-7
27.
Pieters R, Huismans D, Leyva A, Veerman A. 1989. Comparison of the rapid automated MTT-assay with a dye exclusion assay for chemosensitivity testing in childhood leukaemia. Br J Cancer. 59(2):217-220. https://doi.org/10.1038/bjc.1989.44
28.
Twentyman PR, Fox NE, Rees JKH. 1989. Chemosensitivity testing of fresh leukaemia cells using the MTT colorimetric assay. Br J Haematol. 71(1):19-24. https://doi.org/10.1111/j.1365-2141.1989.tb06268.x
29.
Plumb JA, Milroy R, Kaye SB. 1989. Effects of the pH dependence of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide-formazan absorption on chemosensitivity determined by a novel tetrazolium-based assay. Cancer Res. 494435–4440.
30.
Alley MC, Scudiero DA, Monks A, Hursey ML, Czerwinski MJ, Fine DL, Abbott BJ, Mayo JG, Shoemaker RH, Boyd MR. 1988. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 48589–601.
31.
Heo DS, Park JG, Hata K, Day R, Herberman RB, Whiteside TL. 1990. Evaluation of tetrazolium-based semiautomatic colorimetric assay for measurement of human antitumor cytotoxicity. Cancer Res. 503681–3690.
32.
Ruben RL, Neubauer RH. 1987. Semiautomated colorimetric assay for in vitro screening of anticancer compounds. Cancer Treat Rep. 711141–1149..
33.
Rubinstein LV, Shoemaker RH, Paull KD, Simon RM, Tosini S, Skehan P, Scudiero DA, Monks A, Boyd MR. 1990. Comparison of In Vitro Anticancer-Drug-Screening Data Generated With a Tetrazolium Assay Versus a Protein Assay Against a Diverse Panel of Human Tumor Cell Lines. JNCI Journal of the National Cancer Institute. 82(13):1113-1117. https://doi.org/10.1093/jnci/82.13.1113
34.
35.
Slater T, Sawyer B, Sträuli U. 1963. Studies on succinate-tetrazolium reductase systems. Biochimica et Biophysica Acta. 77383-393. https://doi.org/10.1016/0006-3002(63)90513-4
36.
Berridge M, Tan A. 1993. Characterization of the Cellular Reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT): Subcellular Localization, Substrate Dependence, and Involvement of Mitochondrial Electron Transport in MTT Reduction. Archives of Biochemistry and Biophysics. 303(2):474-482. https://doi.org/10.1006/abbi.1993.1311
登录以继续。
如要继续阅读,请登录或创建帐户。
暂无帐户?