傅-克酰基化反应
反应
傅-克酰基化反应是一种芳烃与酰氯或酸酐使用强路易斯酸催化剂的反应。该反应通过亲电芳族取代进行,形成单酰化产物。1,2
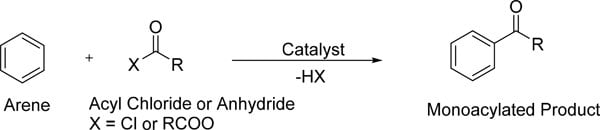
图 1.显示分子结构的傅-克酰基化反应
傅-克酰基化反应机理
傅克酰基化反应包括在路易斯酸和酰氯的氯原子之间形成络合物。通过裂解复合物的C-Cl键形成酰基离子。酰基离子在碳上带正电荷,且共振稳定。该酰基离子充当亲电试剂并与芳烃反应产生单酰化产物(芳基酮)。1
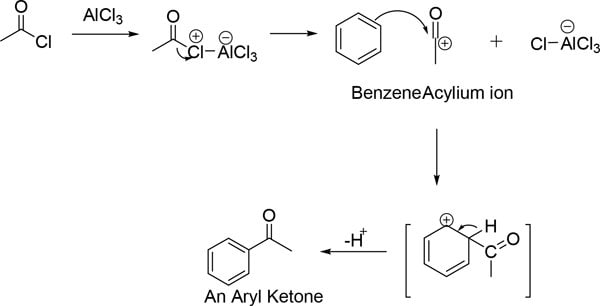
图 2.AlCl3催化傅-克酰基化反应机理(含分子结构)
傅-克酰基化反应是以 Charles Friedel 和 James Mason Crafts 命名的,他们在 1877 年发现了将取代基连接到芳环上的反应。3
注意事项
请参阅产品化学品安全技术说明书,获取危害和安全操作方法相关信息。
应用
傅-克酰基化反应可用于合成:
- 二芳基乙酸衍生物4
- 聚(氧-1,3-亚苯基羰基-1,4-亚苯基)或mPEK5
- 1,5-双(4-氟苯甲酰基)-2,6-二甲基6
- 芳香酮7
- 不对称芳香胺8
- 环酮,如1-茚满酮和1-四氢萘酮9
- 2-乙酰基-6-甲氧基萘,一种合成萘普生的中间体10
最近的研究和趋势
- 聚(4-乙烯基吡啶)负载的三氟甲磺酸已被用作傅克反应中的有效且易于处理的固体超强酸催化剂体系。通过在温和条件下乙醛酸与芳烃的傅-克羟烷基化反应,实现了一锅无溶剂合成各种二芳基乙酸衍生物。4
上述合成方案特点:
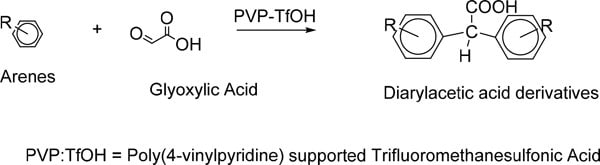
图 3.通过乙醛酸与芳烃的傅-克羟烷基化反应,一锅无溶剂合成二芳基乙酸衍生物
- 基于咪唑的离子液体催化芳族化合物与乙酰氯的傅-克酰基化。11
- 据悉,三氟甲磺酸铒是含有给电子取代基的芳烃的微波辅助傅-克酰基化的良好催化剂。12
- 分子内傅-克酰基化用于直接和短期构建二联苯的ACDE环系统,虎皮楠生物碱是一种新型的交让木属生物碱。13
- 酸促进的多米诺傅-克酰基化反应已用于构建台湾喹啉类的核心6,5,6-ABC三环骨架。它还用于合成二萜(±)-甲萘醌B和(±)-二氯酮。14
- 通过亲电傅-克酰基化缩聚反应合成了含有1,4-亚萘基单元的新型聚(芳醚酮)和聚(芳醚酮砜)。15
- 在SBA-15介孔二氧化硅上接枝的三苯基锡上甲苯与乙酸酐的傅-克酰基化已有报道。16
- 三氟甲磺酸铟在离子液体 1-异丁基-3-甲基咪唑二氢磷酸([i-BMIM]H2PO4)中形成一种有效的绿色催化剂体系,用于芳香族化合物与酸酐的傅-克酰基化反应。17
- 使用酰氯和AlCl3在1,2-二氯乙烷中傅-克酰基化25,27-二烷氧基杯[4]芳烃实验已有报道。18
1.
Fox MA, Whitesell JK. 1994. Organic Chemistry. Boston: Jones and Bartlett.
2.
Li JJ. 2009. Name Reactions: A Collection of Detailed Mechanisms and Synthetic Applications. Springer.
3.
Sartori G, Maggi R. Advances in Friedel-Crafts Acylation Reactions. https://doi.org/10.1201/9781420067934
4.
Prakash GS, Paknia F, Kulkarni A, Narayanan A, Wang F, Rasul G, Mathew T, Olah GA. 2015. Taming of superacids: PVP-triflic acid as an effective solid triflic acid equivalent for Friedel?Crafts hydroxyalkylation and acylation. Journal of Fluorine Chemistry. 171102-112. https://doi.org/10.1016/j.jfluchem.2014.08.020
5.
Baek J, Lyons CB, Tan L. 2004. Grafting of Vapor-Grown Carbon Nanofibers via in-Situ Polycondensation of 3-Phenoxybenzoic Acid in Poly(phosphoric acid). Macromolecules. 37(22):8278-8285. https://doi.org/10.1021/ma048964o
6.
Ohno M, Takata T, Endo T. 1995. Synthesis of a novel naphthalene-based poly(arylene ether-ketone) by polycondensation of 1,5-bis(4-fluorobenzoyl)-2,6-dimethylnaphthalene with bisphenol a. J. Polym. Sci. A Polym. Chem.. 33(15):2647-2655. https://doi.org/10.1002/pola.1995.080331511
7.
de Noronha RG, Fernandes AC, Romão CC. 2009. MoO2Cl2 as a novel catalyst for Friedel?Crafts acylation and sulfonylation. Tetrahedron Letters. 50(13):1407-1410. https://doi.org/10.1016/j.tetlet.2009.01.039
8.
Nordlander JE, Payne MJ, Njoroge FG, Balk MA, Laikos GD, Vishwanath VM. 1984. Friedel-Crafts acylation with N-(trifluoroacetyl)-.alpha.-amino acid chlorides. Application to the preparation of .beta.-arylalkylamines and 3-substituted 1,2,3,4-tetrahydroisoquinolines. J. Org. Chem.. 49(22):4107-4111. https://doi.org/10.1021/jo00196a001
9.
Tran PH, Huynh VH, Hansen PE, Chau DN, Le TN. 2015. An Efficient and Green Synthesis of 1-Indanone and 1-Tetralone via Intramolecular Friedel-Crafts Acylation Reaction. Asian Journal of Organic Chemistry. 4(5):482-486. https://doi.org/10.1002/ajoc.201402274
10.
Kobayashi S, Komoto I. 2000. Remarkable Effect of Lithium Salts in Friedel?Crafts Acylation of 2-Methoxynaphthalene Catalyzed by Metal Triflates. Tetrahedron. 56(35):6463-6465. https://doi.org/10.1016/s0040-4020(00)00610-4
11.
Cai M, Wang X. 2014. Activity of Imidazolium-Based Ionic Liquids as Catalysts for Friedel-Crafts Acylation of Aromatic Compounds. Asian J. Chem.. 26(18):5981-5984. https://doi.org/10.14233/ajchem.2014.16354
12.
Tran PH, Hansen PE, Nguyen HT, Le TN. 2015. Erbium trifluoromethanesulfonate catalyzed Friedel?Crafts acylation using aromatic carboxylic acids as acylating agents under monomode-microwave irradiation. Tetrahedron Letters. 56(4):612-618. https://doi.org/10.1016/j.tetlet.2014.12.038
13.
Wang W, Li G, Wang S, Shi Z, Cao X. 2015. Direct and Short Construction of the ACDE Ring System of Daphenylline. Chem. Asian J.. 10(2):377-382. https://doi.org/10.1002/asia.201403152
14.
Tang S, Xu Y, He J, He Y, Zheng J, Pan X, She X. 2008. Application of a Domino Friedel?Crafts Acylation/Alkylation Reaction to the Formal Syntheses of (±)-Taiwaniaquinol B and (±)-Dichroanone. Org. Lett.. 10(9):1855-1858. https://doi.org/10.1021/ol800513v
15.
Wen H, Wang P, Cheng S, Yan T, Cai M. 2015. Synthesis and characterization of novel organosoluble poly(aryl ether ketone)s and poly(aryl ether ketone sulfone)s containing 1,4-naphthylene units. High Performance Polymers. 27(6):705-713. https://doi.org/10.1177/0954008314557707
16.
Deng Q, Qin Z, Yang Y, Song W. 2015. Synthesis, characterization of triphenyltin grafted on SBA-15 mesoporous silica and its catalytic performance for the synthesis of 4-methylacetophenone. Chinese Journal of Chemical Engineering. 23(2):384-388. https://doi.org/10.1016/j.cjche.2013.12.001
17.
Tran PH, Hansen PE, Hoang HM, Chau DN, Le TN. 2015. Indium triflate in 1-isobutyl-3-methylimidazolium dihydrogen phosphate: an efficient and green catalytic system for Friedel?Crafts acylation. Tetrahedron Letters. 56(17):2187-2192. https://doi.org/10.1016/j.tetlet.2015.03.051
18.
Skácel J, Budka J, Eigner V, Lhoták P. 2015. Regioselective Friedel?Crafts acylation of calix[4]arenes. Tetrahedron. 71(13):1959-1965. https://doi.org/10.1016/j.tet.2015.02.021
登录以继续。
如要继续阅读,请登录或创建帐户。
暂无帐户?