Lyo ECM Gel: A Novel Lyophilized Basement Membrane Extract for 2D and 3D Cell Culture
What is Basement Membrane Extract?
A basement membrane extract, also known as BME, is a complex mixture of extracellular matrix (ECM) proteins, including collagen IV, entactin, laminin, and heparan sulfate proteoglycan, derived from Engelbreth-Holm-Swarm (EHS) mouse sarcoma. BMEs support cells as a surface coating that mimics the natural basement membrane for epithelial and endothelial cells, providing biochemical cues and mechanical stability to cells in vitro.
Researchers use BMEs as scaffolds in traditional 2D and more advanced 3D cell culture systems to establish organoid cultures, model angiogenesis, study tumor invasion, and investigate cell-matrix interactions. Tumor-derived BMEs are often used in stem cell research as well as developmental and cancer biology because they recapitulate the microenvironment for a more physiologically relevant model. However, due to their batch variability and undefined composition, synthetic and recombinant BME alternatives have been developed that show improved consistency and translational potential.
Lyo ECM Gel: A Lyophilized Basement Membrane
Lyo ECM Gel (E1272) is a lyophilized version of ECM gel for cell culture. It provides an adjustable, stable, and easy-to-use material, derived from mouse sarcoma, and offers a biological environment that mimics the natural ECM and supports cell attachment, growth, and function. This product is designed to be reconstituted at user-defined concentrations, offering more flexibility for various biological applications than traditional ECM gels.
Features and Benefits
- Easier to Handle: Room temperature storage and handling.
- Ready When You Need it: Just add water and go.
- Flexibility: Ability to adjust concentration of gel for difficult to culture applications such as organoids.
- Easier Global Shipping/Logistics
How to Use Lyo ECM Gel
Step 1

Grab your Lyo ECM Gel
Step 2

Step 3

Lyo ECM Gel Cell Culture Applications
Angiogenesis Assays
The formation of new capillary blood vessels from pre-existing vasculature, known as angiogenesis, is a highly regulated process that is essential for biological development, wound healing, and tissue regeneration. Dysregulated angiogenesis is a hallmark of a variety of pathologies, including chronic inflammation, ischemic diseases, diabetic retinopathy, and cancer. With angiogenesis assays, researchers can study the cellular and molecular events underlying new blood vessel formation. Common experimental approaches include endothelial cell proliferation and migration assays, tube formation assays, aortic ring and chorioallantoic membrane (CAM) models, as well as in vivo Matrigel® plug and tumor angiogenesis assays.
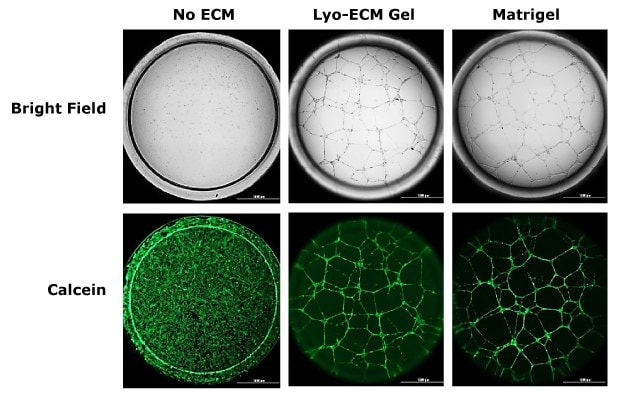
Figure 1. Angiogenesis assays.15K human vein endothelial cells (HUVECs) were seeded on wells coated with reconstituted Lyo ECM Gel or Matrigel® in media containing PMA (50ng/ml) for 24 hrs. Cells stained with Calcein-AM (1mM).
Cellular Invasion and Migration Assays
A fundamental biological process, cell migration drives tissue development, wound repair, disease progression, and immune surveillance. However, aberrant cell migration can contribute to pathological conditions including fibrosis, cancer metastasis, and chronic inflammation. Through migration assays, researchers can evaluate the movement of cells in response to specific stimuli or environmental conditions and obtain qualitative and quantitative insights into cellular dynamics like directionality, mechanisms of motility, and speed. Migration assays can include simple two-dimensional models such as scratch or wound healing assays and Transwell or Boyden chamber assays, and more advanced three-dimensional systems that mimic tissue-like environments.
Total Average of Cell Count
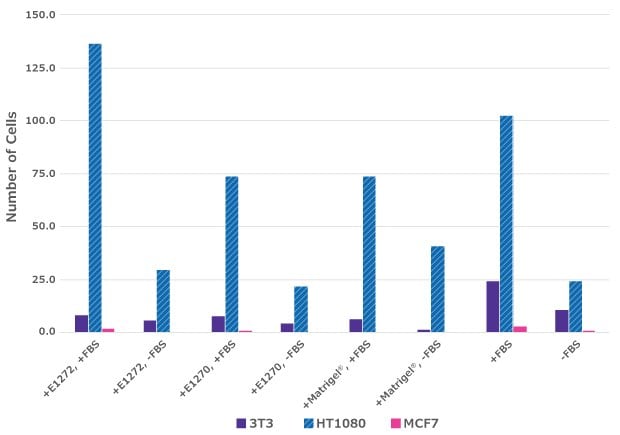
Figure 2. Cell Migration Assays.25K HT1080, NIH3T3 and MCF7 cells were analyzed for cell migration using a Boyden chamber assay technique. Millicell® cell culture inserts (PTEP24H48) were coated with Lyo ECM gel/Matrigel® and +/- FBS was added to culture media to induce migration.
3D Organoid Cultures
Organoid cultures represent advanced, physiologically relevant 3D systems that mimic the structural and functional complexity of native tissues more accurately than conventional 2D cell culture models. One of the most critical factors for successfully generating and maintaining organoids long-term is the extracellular matrix (ECM); Corning® Matrigel® is the most widely used ECM for in vitro organoid culture. Researchers often use “Matrigel® domes”, small, 3D droplets of Matrigel® that are polymerized on the surface of a culture plate, as a scaffold for growing cells or organoids.

Figure 3. Gastrointestinal Organoid Cultures.Human Duodenum intestinal organoids (SCC322) were cultured in organoid expansion media in either Lyo ECM Gel or Matrigel® domes for 5 passages. No difference in morphology and cell health was seen between the two conditions after 5 passages.
Neurite Outgrowth Assays
Neurites, which can include axons and dendrites, are projections that extend from the cell body of neurons; their growth is a fundamental step in neurodevelopment, regeneration, and synaptic network formation. Neurite outgrowth assays are widely used experiments for the study of processes that govern neuronal differentiation, axon and dendrite formation, and neural connectivity. By quantifying neurite initiation, elongation, branching, and guidance, neurite outgrowth assays provide researchers crucial insight into the molecular mechanisms that regulate neuronal plasticity and repair.
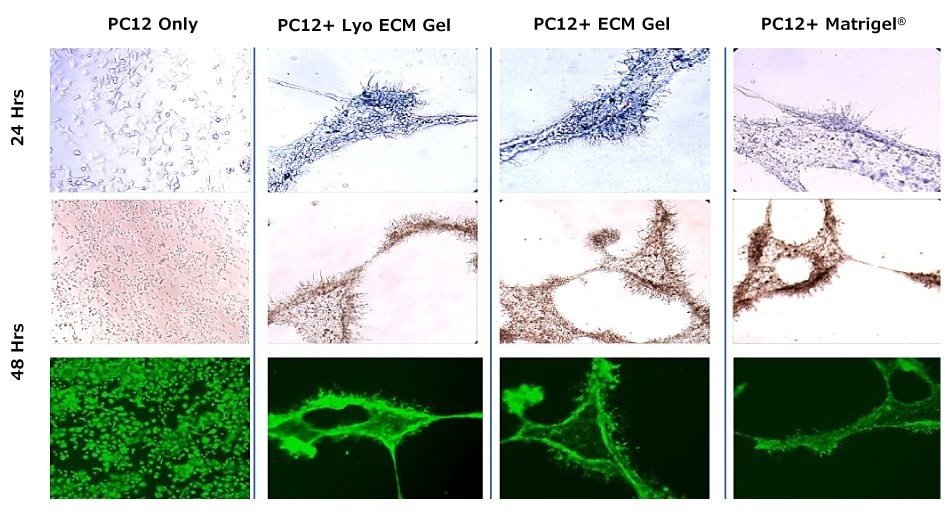
Figure 4. Differentiation of PC12 cells.PC12 neural cells were grown in 24 well plates on polymerized ECM Gel, Lyo ECM Gel or Matrigel® for 48 hrs. PC12 alone without matrixes was used as a negative control. After 48 hours differentiation neurite formation was detected in ECM gel conditions.
Related Products
如要继续阅读,请登录或创建帐户。
暂无帐户?