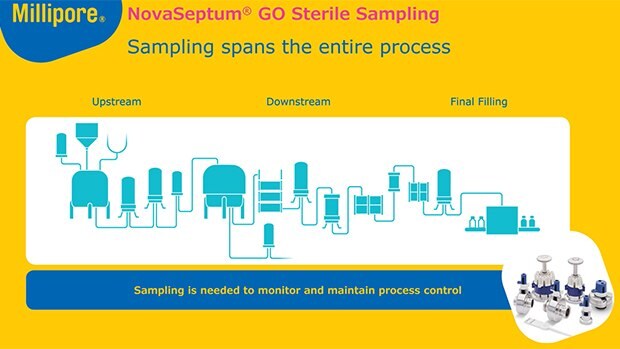
最终无菌过滤和灌装
最终灭菌过滤或批量过滤是单克隆抗体(mAb)生产过程中的关键步骤,因为它能确保从药物产品中去除细菌污染物。多份监管指导文件描述了过滤操作的具体注意事项,包括对过滤器完整性测试的要求和建立过滤系统与工艺流兼容性的期望。
最终灌装涉及在高度受控的灌装环境中将无菌过滤后的药物产品转移到小瓶、注射器或其他容器中,以保持无菌状态并降低产品污染的风险。
- Millipore® 过滤器
- Millipore® 一次性使用解决方案(2D/3D 一次性使用组件和储存系统、最终灌装组件、连接器)
- Emprove 档案以支持工艺优化和安全风险评估,并提供详细的萃取物简介
- 密理博® 设备和系统
- Millipore® .nbsp;设备和amp;系统(完整性测试仪、滤器外壳<)
- Millipore® 取样解决方案(离线系统)
- Millipore® ;服务(一次性使用和过滤器验证、系统服务、设备安装、系统鉴定、系统培训)
- Millipore® ;自动化和分析软件
- BioReliance® Final Product Release Testing
- Millipore® CTDMO Services
相关资料
相关类别
相关资源
- Application Note: Validation Master Plan For Filtration Systems Used In Aseptic Processing
Summarizes best practices for validating performance of critical filtration systems used in aseptic processing and details on the tests needed to meet global regulatory requirements.
- Tech note: Millipak® Final Fill Filters Reduce Contamination Risks and Simplify Filtration System Design and Operation
This tech note summarizes the results of microbial challenge studies that confirm the aseptic multi-purpose port (AMPP) prevents microbial contamination from entering the flow path.
- Application Note: Integrity Test Troubleshooting – Beyond Rewet and Retest
In this Application Note, we consider all the potential sources of test error and apply a logical approach to resolution and retesting.
- White Paper: Aseptic Process Sampling Risk Mitigation – A Regulatory Perspective
There are significant consequences associated with microbial contamination during biopharmaceutical manufacturing. Contamination increases risks for the operator, the company, and potentially the patient, all of which can result in significant negative impact.
- Application Note: Microbial Integrity of NovaSeptum® Sampling Systems upon Performance of Multiple Sampling Actuation
The NovaSeptum® and NovaSeptum® GO sampling systems are a family of products designed for single-use sterile sampling throughout the biomanufacturing process.
- Application Note: How Millipak® Barrier and Millidisk® Barrier Filters Streamline Final Sterile Filtration
These barrier filters streamline filter venting and flushing during integrity testing of critical sterilizing filters.
探索我们的解决方案
如要继续阅读,请登录或创建帐户。
暂无帐户?